Method for in-situ precipitation and separation of arsenic in copper electrolyte
A copper electrolyte and in-situ precipitation technology, which is applied in the direction of electrolysis components, electrolysis process, instruments, etc., can solve the problems of electrolysis process influence, large addition, increase the processing space and cost of purified electrolyte, and achieve high-efficiency settlement and reduce The effect of the nucleation barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
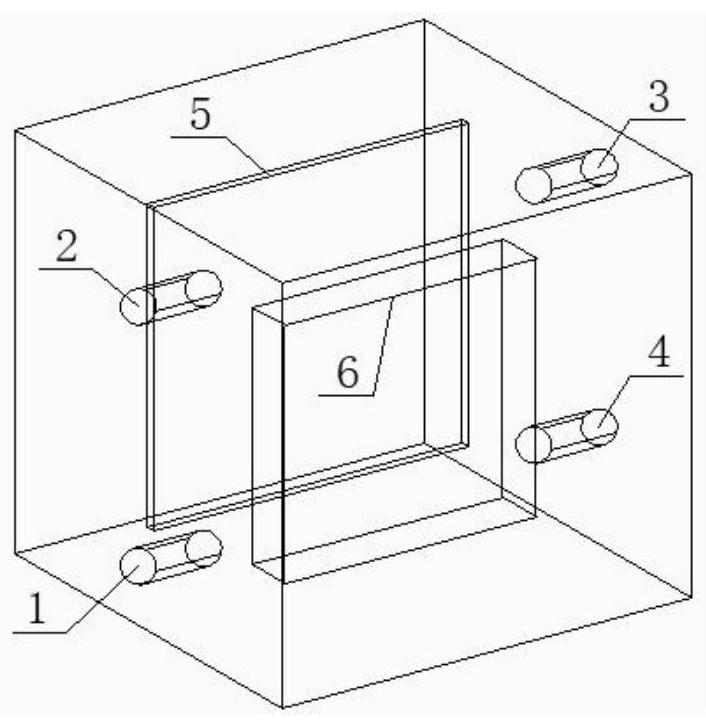
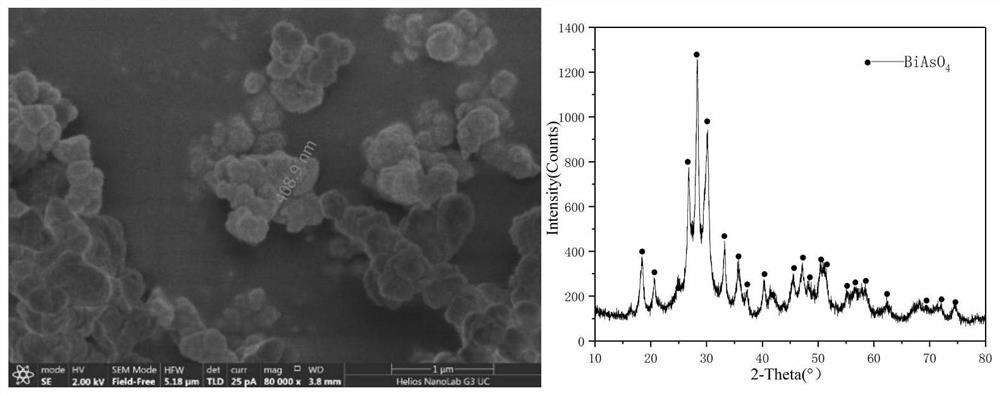
[0028] Taken from a copper electrolyte in a smelter, the concentrations of As in it are 3407mg / L, Sb382mg / L, and Bi787mg / L. Take 2L of the electrolyte in the electrolytic cell, and place it in a water bath at a constant temperature of 70°C, use a peristaltic pump to control the circulation rate of the electrolyte to 50ml / L, and the circulation mode is bottom-in and top-out / parallel plates Liquid, the distance between the cathode and anode plates is controlled to be 2.5cm, and the distance between the center of the liquid inlet and the anode plate is 2.2cm, and the current density is 500A / m 2 Carry out the reaction, after the reaction is carried out for 4 hours, add the seed crystal from the liquid inlet pipe through the peristaltic pump, the amount of the added seed crystal is 1g, collect the precipitate produced after two days of reaction and do corresponding SEM and XRD detection, the detection results are as follows image 3 shown. From the test results, it can be seen tha...
Embodiment 2
[0030] Taken from a copper electrolyte in a smelter, the concentrations of As in it are 3407mg / L, Sb382mg / L, and Bi787mg / L. Take 2L of the electrolyte in the electrolytic cell, and place it in a water bath at a constant temperature of 70°C, use a peristaltic pump to control the circulation rate of the electrolyte to 50ml / L, and the circulation mode is bottom-in and top-out / parallel plates Liquid, the distance between the cathode and anode plates is controlled to be 2.5cm, and the distance between the center of the liquid inlet and the anode plate is 2.2cm, and the current density is 500A / m 2 Carry out the reaction, after the reaction is carried out for 4 hours, add the seed crystal from the liquid inlet pipe through the peristaltic pump. The amount of the added seed crystal is 2g. After two days of reaction, the precipitate produced is collected and tested by SEM and XRD. The test results are as follows: Figure 4 shown. From the test results, it can be seen that the precipit...
Embodiment 3
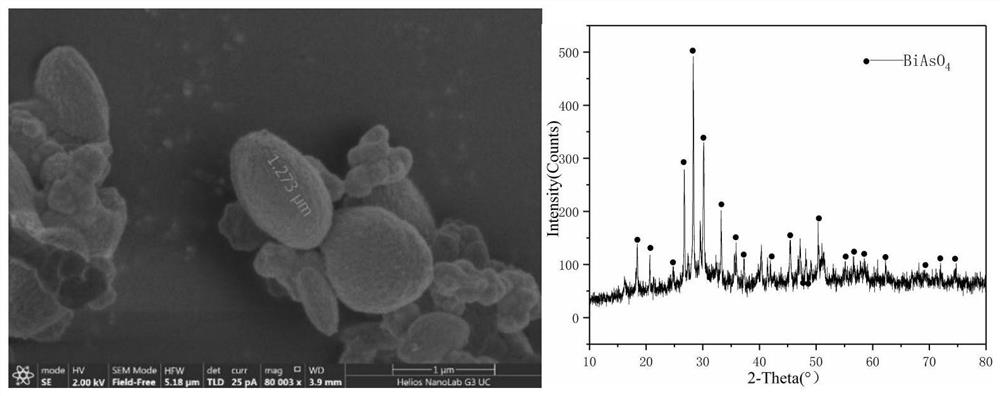
[0032] Taken from a copper electrolyte in a smelter, the concentrations of As in it are 3407mg / L, Sb382mg / L, and Bi787mg / L. Take 2L of the electrolyte in the electrolytic cell and place it in a water bath at a constant temperature of 70°C. Use a peristaltic pump to control the circulation rate of the electrolyte to 50ml / L. The distance between the anode plates is 2.5cm, and the distance between the center of the liquid inlet and the anode plate is 0.3cm, and the current density is 500A / m 2 Carry out the reaction, after the reaction is carried out for 4 hours, add the seed crystal from the liquid inlet pipe through the peristaltic pump. The amount of the added seed crystal is 2g. After two days of reaction, the precipitate produced is collected and tested by SEM and XRD. The test results are as follows: Figure 5 shown. From the test results, it can be seen that the precipitate obtained under this condition has a single phase, mainly BiAsO 4 Substance; ellipsoidal particles, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com