NMN medical composition for AD treatment
A composition and drug technology, applied in the field of medicine, can solve problems such as high drug concentration, high manufacturing cost, and limited clinical promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 alpine edelweiss extract
[0033] Accurately weigh 1000g of alpine edelweiss plant cell culture (moisture content above 90%), after fully suspending, add 2000mL of 95% ethanol at a material-to-liquid ratio of 1:2 (m / v), stir and mix, and ultrasonically treat 15min (ultrasonic power: 300w, ultrasonic temperature: 25°C); add 8L of pure water to the ultrasonically broken wall treatment product, and extract at a high temperature of 80-120°C for 1.5h; centrifuge the extracted product at 10,000g for 10min to take the supernatant, The supernatant was concentrated under reduced pressure and then freeze-dried to obtain the edelweiss alpine extract.
Embodiment 2
[0034] The preparation of embodiment 2 pharmaceutical compositions
[0035] After accurately weighing 4g of NMN and 80g of alpine edelweiss extract and mixing them uniformly in equal proportions, the pharmaceutical composition is obtained.
Embodiment 3
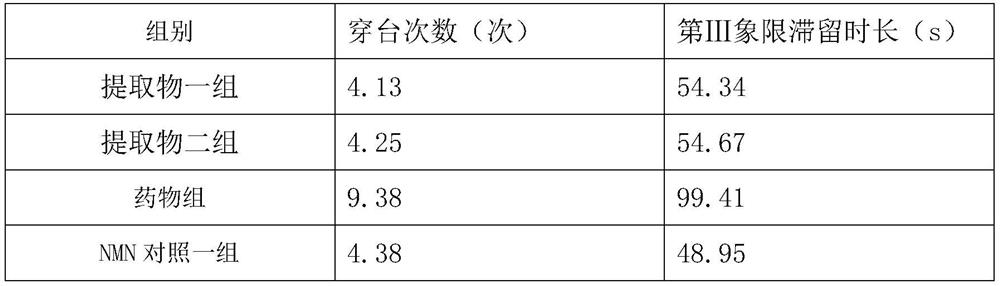
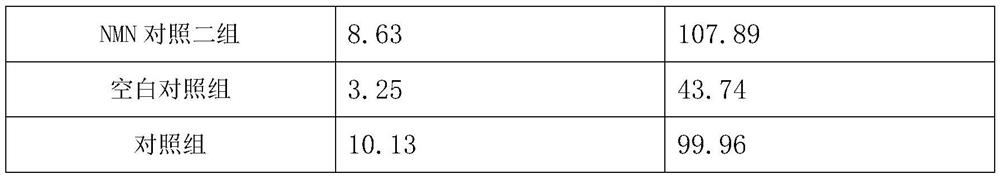
[0036] Example 3 Study and cognitive intervention experiment of pharmaceutical composition on Tg2576 transgenic mice
[0037] Experimental Materials:
[0038] 1) Experimental animals: 48 8-week-old Tg2576 transgenic male mice, 8 8-week-old C57BL / 6J male mice with the same genetic background, all purchased from Saiye (Suzhou) Biotechnology Co., Ltd. Housing group temporary care.
[0039] 2) Test drugs:
[0040] The pharmaceutical composition prepared in Example 2 of the present invention;
[0041] NMN: Bangtai Bioengineering (Shenzhen) Co., Ltd.
[0042] 3) Main experimental instruments
[0043] Morris water maze system (including camera and analysis system) was purchased from Shanghai Ruanlong Technology Development Co., Ltd.
[0044] 4) Experimental method:
[0045] grouping:
[0046] 48 Tg2576 transgenic male mice were randomly divided into 6 groups, extract group 1, extract group 2, drug group, NMN control group, NMN control group 2 and blank control group, 8 mice in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com