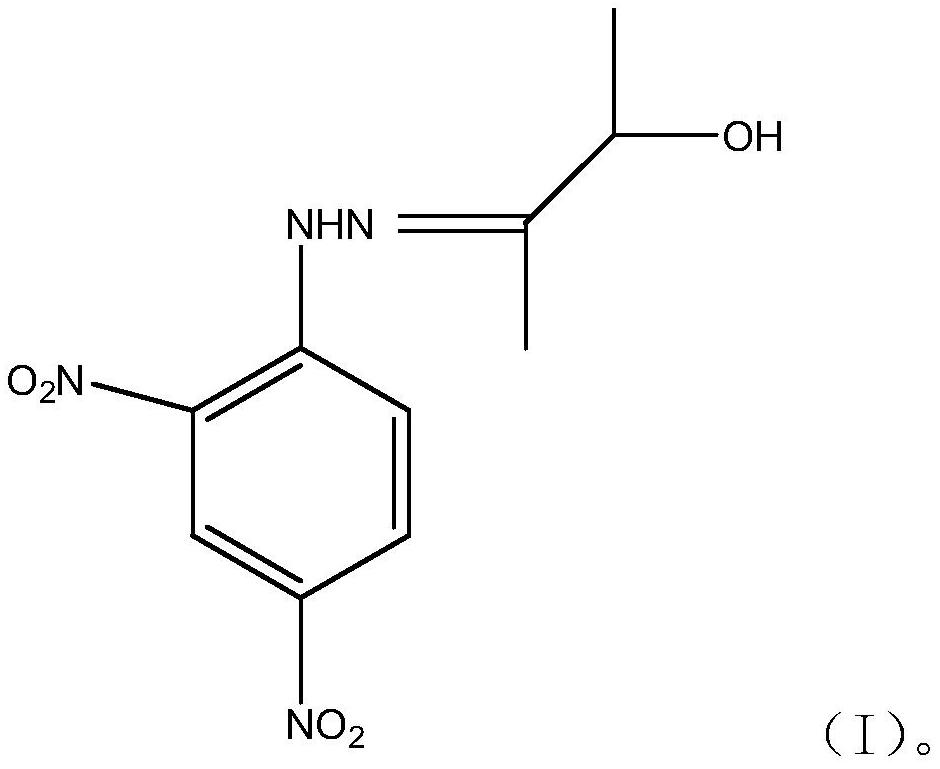
3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone and method for detecting acetoin content in exhaled breath by using same
A technology of dinitrophenylhydrazone and dinitrophenylhydrazine, which is applied in the field of acetoin content and 3-hydroxy-2-butanone-2, can solve the problems of unquantifiable detection content and complex sample components, etc. Achieve the effect of convenient and safe operation and processing, simple synthesis route and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
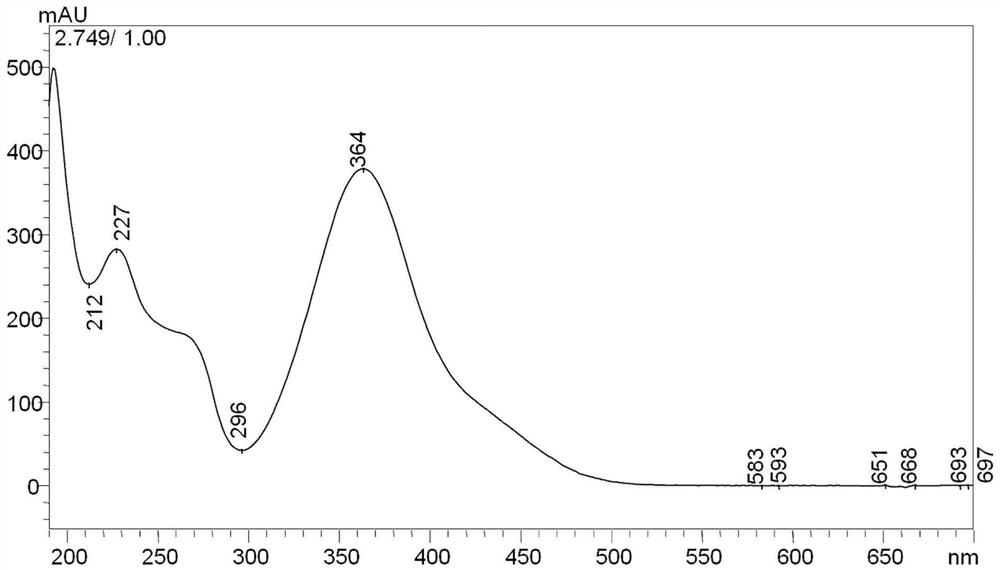
[0040] Prepare a certain amount of 2,4-dinitrophenylhydrazine (DNPH) reagent in a beaker, add acetonitrile to dissolve, make DNPH supersaturated solution, transfer the upper layer solution to another beaker (the upper layer solution is the saturated DNPH solution ), in this saturated DNPH solution, add concentrated hydrochloric acid (concentrated hydrochloric acid is laboratory concentrated hydrochloric acid, content between 36%-38%), obtain acidic saturated 2,4-dinitrophenylhydrazine solution, acidic saturation 2, 4-Dinitrophenylhydrazine solution contains 0.005% volume fraction of hydrogen ions.
[0041] Add 3-hydroxy-2-butanone into acetonitrile and dissolve it to form a 3-hydroxy-2-butanone solution. The acetonitrile contains 0.005% volume fraction of hydrogen ions. Put the above acidic saturated DNPH solution at a constant temperature of 60°C In a water bath, after the temperature of the acidic saturated DNPH solution reaches 60°C, add 3-hydroxy-2-butanone solution into t...
Embodiment 2
[0051] The molar ratio of the amount of 3-hydroxy-2-butanone added to the amount of 2,4-dinitrophenylhydrazine is 1:2, and the others are the same as in Example 1. The yield of the reaction product was measured to be 97.6%, and the purity of the purified 3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone was 99.42%.
Embodiment 3
[0053] The molar ratio of the amount of 3-hydroxy-2-butanone added to the amount of 2,4-dinitrophenylhydrazine is 1:3, and the others are the same as in Example 1. The yield of the reaction product was measured to be 99.7%, and the purity of the purified 3-hydroxy-2-butanone-2,4-dinitrophenylhydrazone was 98.83%.
[0054] Preparation of Sorbent Sampling Tubes
[0055] 2,4-dinitrophenylhydrazine (DNPH) is configured with acetonitrile into a coating solution containing 3% of the derivatizing reagent; then 200 mg of stationary phase filler is filled on a blank adsorption column; finally the coating solution is coated with On the adsorption column filled with stationary phase, the coating method is carried out by direct coating method. Rinse the blank adsorption column with acetonitrile before coating, and remove the rinsed acetonitrile reagent by the negative pressure solid phase extraction system, then apply 3% coating solution directly on the adsorption column, and use negativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com