Artificial antigen presenting cells and methods of use
A technology of artificial antigens and cells, applied in the fields of biochemical equipment and methods, animal cells, cancer antigen components, etc., can solve problems such as expensive, time-consuming, and limited standardization of treatment plans.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1090] Formulations of the pharmaceutical compositions described herein can be prepared by any method known or later developed in the art of pharmacology. In general, such preparation methods include the step of bringing into association the active ingredient with the carrier or one or more other accessory ingredients, and then, if necessary or desired, shaping or packaging the product into desired unit-dose or multi-dose units.
[1091] Although the description of pharmaceutical compositions provided herein is primarily directed to pharmaceutical compositions suitable for ethical administration to humans, those skilled in the art will appreciate that such compositions are generally suitable for administration to all species of animals. Modifications of pharmaceutical compositions suitable for human administration in order to adapt the compositions for administration to various animals are well known and can be devised and performed by only ordinary, if any, experimentation by ...
Embodiment 1
[1124] Example 1. Generation of Erythroid Cells Genetically Engineered to Express MOG-MHCII-GPA Fusion Protein
[1125] result
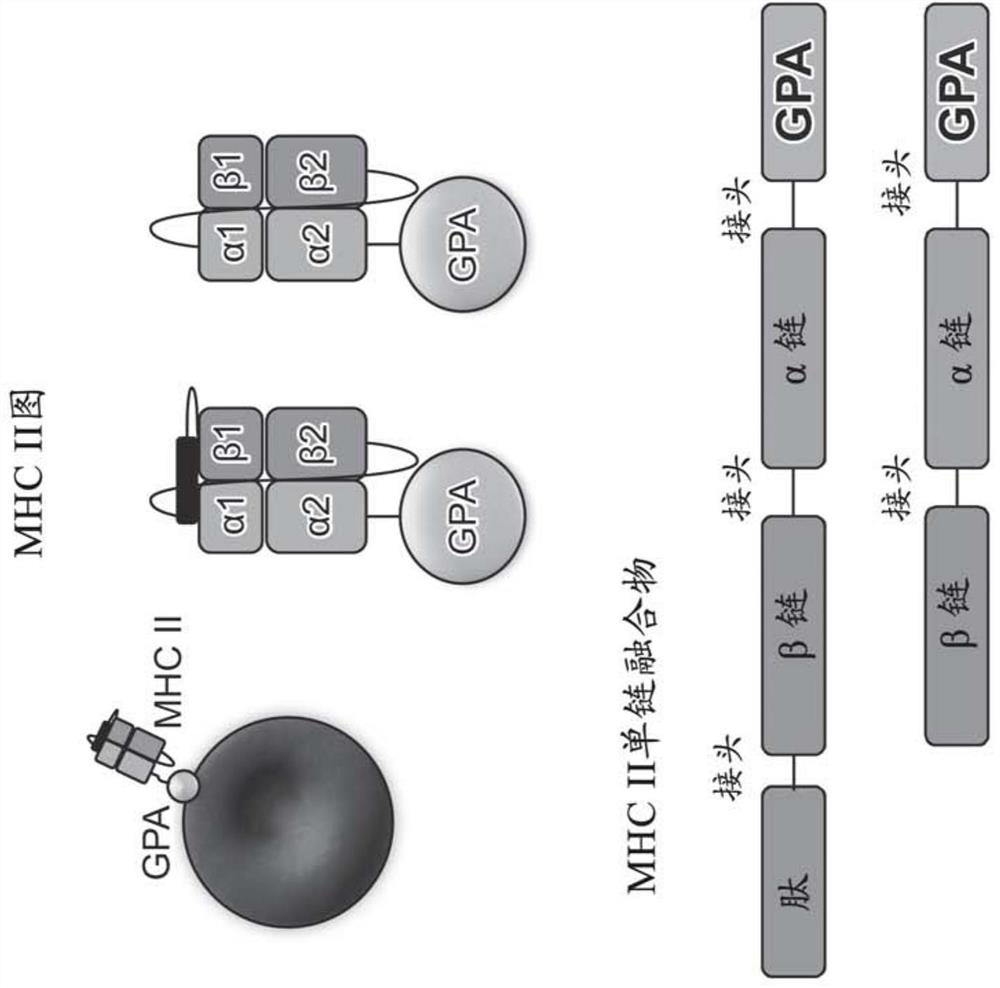
[1126] Erythroid cells were transduced to express a fusion protein comprising an exogenous antigenic peptide (myelin oligodendrocyte glycoprotein (MOG)), fused with an exogenous antigen-presenting polypeptide (especially MHCII), and GPA spanning Fusion of a membrane domain (GPA) that acts as a membrane anchor (MOG-MHCII-GPA). Figure 1A A schematic diagram of the design used to express MOG peptide and MHCII as a single-chain fusion is shown, wherein the exogenous peptide (MOG) is linked to the MHCIIβ chain linked to the MHCIIα chain linked to the GPA membrane anchor. Cell culture and transduction were performed as described in the "Methods" section below to generate erythroid cells expressing MOG presented by MHCII on the cell surface anchored with the GPA transmembrane domain.
[1127] Conjugation of allophycocyanin (APC)-labeled or phycoerythrin (...
Embodiment 2
[1137] Example 2. Generation and in vitro validation of erythroid cells genetically engineered to co-express MOG-MHCII-GPA fusion proteins and co-inhibitory polypeptides
[1138] result
[1139] As described in Example 1, erythroid cells were transduced to express a fusion protein comprising exogenous antigenic peptide (MOG), fused to exogenous antigen-presenting polypeptide (MHCII), and GPA transmembrane domain (GPA ) fusion (MOG-MHCII-GPA). Erythroid cells were co-transduced to additionally express the exogenous co-inhibitory peptide PD-L1. Cell culture and transduction were performed as described in the Methods section below to generate erythroid cells expressing MOG presented by MHCII on the cell surface, anchored with the GPA transmembrane domain, and co-expressing PD-L1.
[1140] As also described in Example 1, the incorporation of APC-labeled or PE-labeled anti-MHCII antibodies was used to verify the expression of MHCII antigen-presenting peptides in engineered erythr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com