Compound with TDO and IDO1 dual inhibitory activity, and application of compound in preparation of drugs for treating neurodegenerative diseases
A compound and pharmaceutical technology, applied in the field of chemical medicine, can solve problems such as inhibition of killing, stagnation, and reduction of tryptophan concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
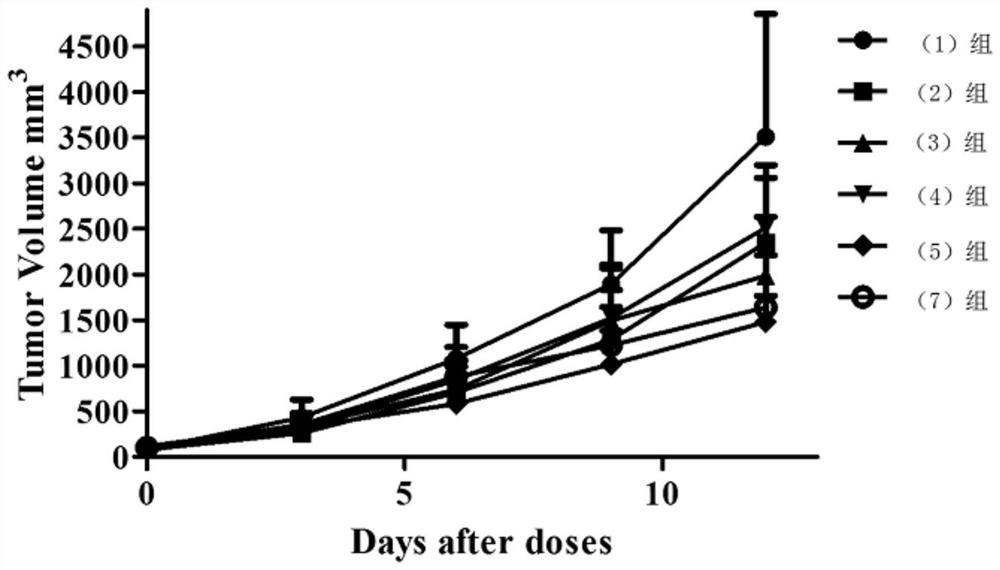
Image
Examples
preparation example Construction
[0087] 1 Preparation of Intermediate A2
[0088] Compound A1 (5.0g, 22.1mmol) was dissolved in 30mL of toluene, DPPA (7.9g, 28.7mmol), Et3N (6.2mL, 44.2mmol) was added under stirring, benzyl alcohol (6.9mL, 66.2mmol) was added after stirring for 10min . After the addition was completed, the temperature was raised to 110°C for reflux. After 3 hours, the reaction was complete, the reaction liquid was concentrated under reduced pressure to remove the reaction solution, the residue was diluted with water, extracted three times with EA, the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. The crude product was purified by column chromatography to obtain light yellow solid A2 (6.22g, 18.8mmol), with a yield of 85%.
[0089] 2 Preparation of Intermediate A3
[0090] A2 (100mg, 0.3mmol) was dissolved in a mixed solvent (methanol:dioxane=1:1), iron powder (51mg, 0.9mmol) and 10N conc...
Embodiment 1
[0150] Example 1, Compounds TA-1, TA-14, TA-15, TA-16, TA-16-a, TA-16-b, TA-16-c, TA-17, TB-1, TB-4 , TB-6, TB-35, TB-36 synthesis
[0151]
[0152] Among them, the structure of TA-16 is: The structure of TA-16-a is: The structure of TA-16-b is: The structure of TA-16-c is:
[0153] Dissolve amines (1.0 equivalents) and different substituted aldehydes (1.2-2.0 equivalents) in dichloromethane (DCM), add dihydropyridine (1.4 equivalents) and an appropriate amount of 4A molecular sieves, drop trifluoroacetic acid (TFA , 0.5 times equivalent), reflux at 40°C for 12 hours. Refer to the method of patent CN108689936A reductive amination for synthesis. Yield: 20%-80%.
Embodiment 2
[0154] Example 2 Compounds TA-4, TA-5, TB-17, TB-26, TB-28, TB-29, TB-30, TB-31, TB-32, TB-33, TB-34, TB- Synthesis of 37
[0155]
[0156] Synthesized with reference to Example 1, the synthetic yield: 17%-88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com