Anti-her2 antibody or antigen-binding fragment thereof, and chimeric antigen receptor comprising same
A technology that combines fragments and antibodies, applied in receptors/cell surface antigens/cell surface determinants, antibody medical components, antibody mimics/scaffolds, etc. Sexual decline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1: Development of anti-HER2 antibodies
[0189] To develop antibodies, the extracellular domain (ECD) of the HER2 protein was produced using animal cells. Using HindIII and BamHI restriction enzymes, the hinge region and Fc region of human IgG1 (CH 2 -CH 3 ) bound to the C-terminus of the ECD was cloned into pCEP4 (Invitrogen, catalog # V044-50). Then, the cloned vector was transiently transformed into FreeStyle 293F (Invitrogen, catalog number R790-07) cells using polyethyleneimine (Polyscience Inc., cat. No. 20078-028) to purify the cloned vector from cell culture. Purified protein was quantified using a protein assay dye (Bio-Rad, cat. no. 500-0006), and its concentration and purity were investigated by Coomassie brilliant blue staining after SDS-PAGE. 100 μg of protein antigen was mixed with Freund's adjuvant (Sigma, Cat. No. F5506), and injected intraperitoneally into BALB / c mice (Dae Han Bio). Two weeks later, 100 μg of antigen diluted in PBS was furt...
Embodiment 2
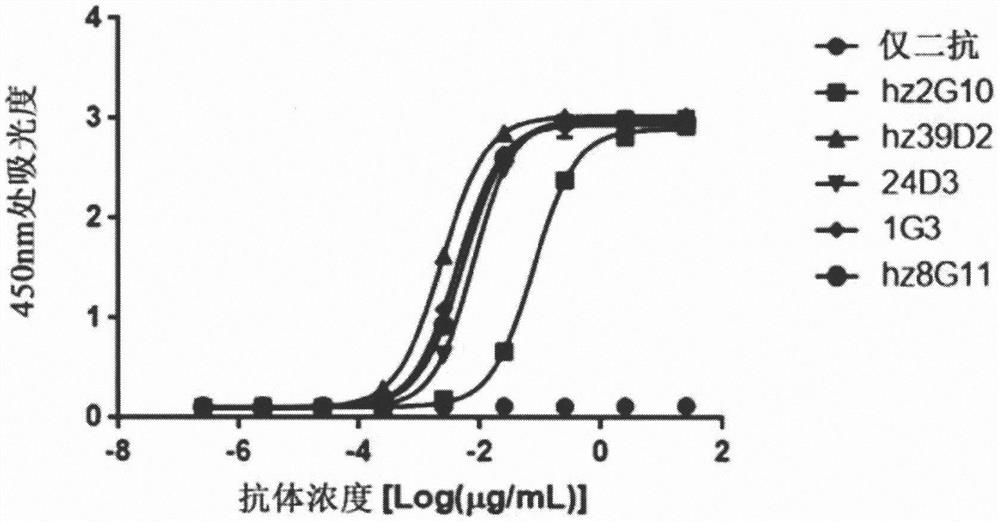
[0197] Example 2: Verification of the binding site of the developed HER2 protein antibody
[0198] The binding sites of the five selected antibodies (hz2G10, hz39D2, 24D3, 1G3, hz8G11) to the extracellular domain (ECD) of HER2 protein were verified by ELISA. For ELISA, extracellular domains (ECDs) of ERBB family proteins were produced using animal cells and used as antigens. Specifically, the hinge region and Fc region (CH 2 -CH 3 ) bound to the C-terminus of the ECD was cloned into pCEP4 (Invitrogen, catalog # V044-50). Then, the cloned vector was transiently transformed into FreeStyle 293F (Invitrogen, catalog number R790-07) cells using polyethyleneimine (Polyscience Inc., cat. 20078-028) Purification of HER2-ECD DI Fc, HER2-ECDDH Fc, HER2-ECD DIII Fc, HER2-ECD DIV Fc and HER2-ECD Fc fusion proteins from cell culture. Purified protein was quantified using a protein assay dye (Bio-Rad, cat. no. 500-0006), and its concentration and purity were investigated by Coomassie br...
Embodiment 3
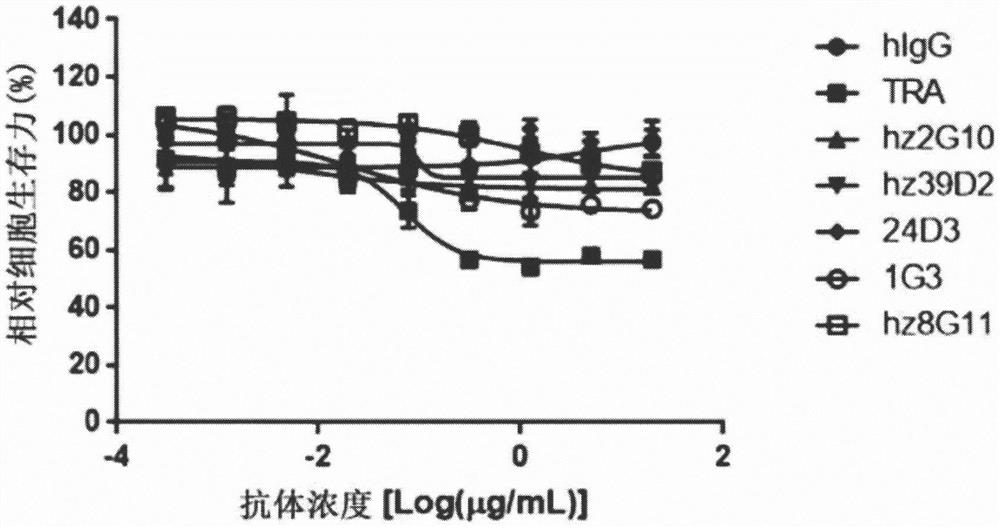
[0203] Example 3: Comparison of the inhibitory effect of the developed antibodies on the growth of breast cancer cells
[0204] Cell viability was analyzed by treating HER2-overexpressing SKBR3 breast cancer cells or HER2-nonexpressing breast cancer cells with MCF-7 alone or in combination with trastuzumab. For combined administration, the developed antibody and trastuzumab were mixed in a 1:1 weight ratio. SKBR3 cells (Korean Cell Line Bank, Cat. No. 30030, 5000 cells / well) and MCF-7 cells (ATCC, Cat. No. HTB22, 5000 cells / well) were aliquoted onto 96-well plates and cultured for 24 hours. After treatment with the purified antibody at a final concentration of 20 μg / mL, the cells were further cultured for 4 days. To measure cell viability, CCK-8 (Dojindo, catalog number CK-04-13) was added to a final concentration of 10%, and absorbance was measured after treatment at 37°C for 3 hours. Relative cell viability was calculated relative to the absorbance of untreated wells as 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com