Lupeol pyridine quaternary ammonium salt derivative, preparation method and application thereof
A technology of pyridine and quaternary ammonium salts of soy alcohol is applied in the field of lupin alcohol pyridine quaternary ammonium salt derivatives and their preparation, which can solve the problems of limited anti-tumor research, unsatisfactory dissolution effect, easy precipitation of drug crystals, and the like, and achieves significant inhibition of effect, obvious anti-cancer effect, significant R&D potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
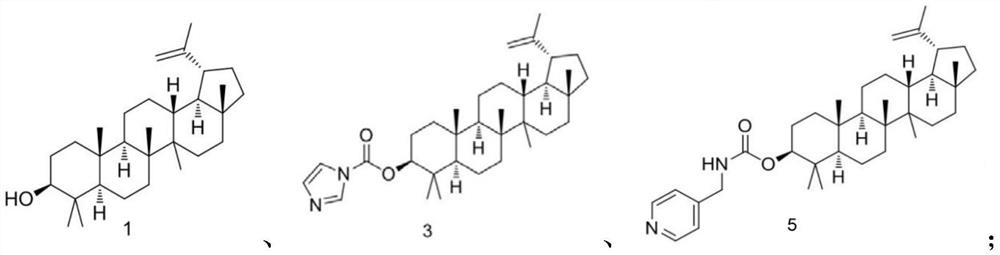
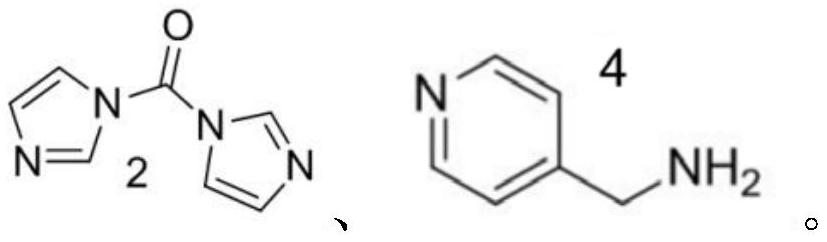
[0039] Embodiment 1: Preparation of compound A (lupeyl alcohol pyridinium quaternary ammonium salt derivative):
[0040]
[0041] Compound 1 (1.28g, 3.0mmol) was dissolved in 30mL of dichloromethane (DCM), compound 2 (0.53g, 3.3mmol) was added, and reacted at room temperature for 2h. TLC monitored that compound 1 was completely reacted. Add 30 mL of dichloromethane to dilute the reaction solution, wash twice with saturated brine, 30 mL each time, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness to obtain 1.42 g of crude compound 3, which was not further processed. purified and used directly in the next step.
[0042] The crude compound 3 was dissolved in 30 mL of dichloromethane, compound 4 (0.39 g, 3.6 mmol) was added thereto, and the reaction was stirred at 40 degrees for 17 h. After the reaction was completed, cool to room temperature, add 30 mL of dichloromethane to dilute the reaction solution, wash twice with satura...
Embodiment 2
[0046] Example 2 Compound A of the present invention inhibits human tumor cell proliferation test
[0047] 1. Drug dissolution: lupeol was dissolved in warm absolute ethanol and DMSO at a ratio of 1:1 to prepare a total solution of 7811.57 μM; compound A of the present invention was dissolved in DMSO to prepare a total solution of 14228.6 μM. Dilute with complete medium to the corresponding working concentration for experiments.
[0048] 2. Cell culture: 16 kinds of tumor cell lines used in the experiment (bladder cancer 5637 cells, kidney cancer 786-O cells, neuroblastoma SK-N-SH cells, liver cancer Huh7 cells, nasopharyngeal carcinoma Cne2 cells, prostate cancer PC3 cells , colon cancer Colo205 cells, colon cancer DLD-1 cells, colon cancer HCT-116 cells, colon cancer HT-29 cells, colon cancer Lovo cells, colon cancer RKO cells, colon cancer SW480 cells, colon cancer SW620 cells, colon cancer Caco2 cells, colon cancer THC-8307 cells; 16 kinds of cell lines are conventional c...
Embodiment 3
[0057] Example 3 The toxicity test of compound A of the present invention to normal human epithelial cells
[0058] 1. Drug dissolution: the compound A of the present invention was dissolved in DMSO, prepared into a total solution of 14228.6 μM, and diluted with complete medium to the corresponding working concentration for experiments.
[0059] 2. Cell culture: human prostate epithelial RWPE-1 cells, liver HL-7702 cells, colonic epithelial HCoEpiC cells, nasopharyngeal epithelial NP69 cells, breast epithelial MCF-10A cells and alveolar epithelial HPAEpiC cells and other 6 strains of normal epithelial cells (all were Conventional commercially available cell lines) were placed in the corresponding medium containing 10% fetal bovine serum (FBS) or without FBS, and kept at 37°C, saturated humidity, and a volume fraction of 5% CO 2 Cultured in an incubator, the corresponding media for various cells are shown in Table 3.
[0060] Table 3 The culture medium of each normal cell
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com