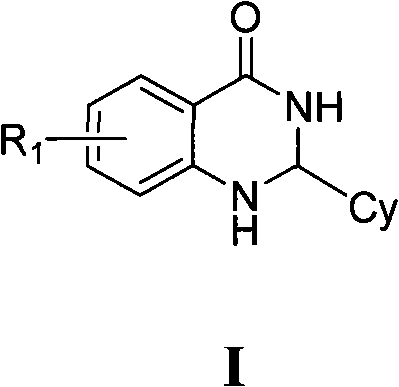
2,3-dihydro-4(1H)- quinazolone derivative and application thereof
A quinazolone and derivative technology, which can be used in drug combinations, cardiovascular system diseases, respiratory system diseases, etc., and can solve the problems of poor tumor curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1, 2-(4,5-dibromo-2-thienyl)-6-nitro-2,3-dihydroquinazolin-4(1H)-one
[0058]
[0059] step:
[0060] Sequentially weigh 5-nitro-2-aminobenzamide (0.181g, 1mmol) and 4,5-dibromo-2-thiophene aldehyde (0.270g, 1mmol) in a 5mL round bottom flask, add 3mL formic acid, room temperature After stirring, a large amount of yellow solids were precipitated. After 5 minutes, TLC detected that the reaction was complete. Stopped the reaction, filtered the yellow solids, and dried them in vacuum at 40° C. for 6 hours to obtain 0.380 g of yellow solids. The yield was 88%, and the purity by HPLC was 98.29%, m.p. : 261-263°C.
[0061] 1 H NMR (DMSO-d 6, ppm) δ: 9.03 (1H, d, J = 1.2Hz), 8.73 (1H, s), 8.42 (1H, d, J = 2.7Hz), 8.15 (1H, dd, J = 9.0, 3.0Hz), 7.15 (1H, d, J = 0.6Hz), 6.89 (1H, d, J = 9.0Hz), 6.27 (1H, t, J = 2.4Hz)
[0062] EI-MS (m / z): 431 (M +. C 12 h 7 79 Br 2 N 3 o 3 S, 50), 352 (M- 79 Br, 55), 192(80), 165(100), 146(30), 119(30)
Embodiment 2
[0063] Example 2, 2-(4-bromo-2-thienyl)-6-nitro-2,3-dihydroquinazolin-4(1H)-one
[0064]
[0065] The operation steps are the same as in Example 1, yellow solid, m.p.: 243-247°C
[0066] 1 H NMR (DMSO-d 6 , ppm) δ: 8.99 (1H, s), 8.72 (1H, s), 8.42 (1H, d, J=3.0Hz), 8.14 (1H, dd, J=9.0, 2.4Hz), 7.64 (1H, d , J=1.8Hz), 7.16 (1H, d, J=1.2Hz), 6.88 (1H, d, J=9.0Hz), 5.29 (1H, t, J=2.4Hz)
[0067] EI-MS (m / z): 353 (M + .C 12 h 8 79 BrN 3 o 3 S, 100), 323 (M- 79 Br, 80), 274(40), 192(29), 165(85), 146(15), 134(16), 119(25), 90(45)
Embodiment 3
[0068] Example 3, 2-(4-bromo-2-thienyl)-7-chloro-2,3-dihydroquinazolin-4(1H)-one,
[0069]
[0070] step:
[0071] Sequentially weigh 4-chloro-2-aminobenzamide (0.181g, 1mmol) and 4,-bromo-2-thiophene aldehyde (0.270g, 1mmol) in a 5mL round bottom flask, add 5mL of ethanol, stir at room temperature, there is A large amount of white solids precipitated out, and the reaction was stopped after the completion of the reaction by TLC, concentrated under reduced pressure, and subjected to silica gel column chromatography to obtain 0.380 g of white solids with a yield of 88%, m.p.: 201-202°C.
[0072] 1 H NMR (DMSO-d 6 , ppm) δ: 8.67 (1H, s), 7.62 (1H, s), 7.59 (2H, s), 7.12 (1H, s), 6.81 (1H, s), 6.73 (1H, dd, J=8.4, 1.2Hz), 6.06 (1H, s) EI-MS (m / z): 342 (M +. C 12 h 8 79 Br 35 ClN 2 OS, 44), 263 (M- 79 Br, 23), 181(45), 154(100), 126(40)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com