Application of bone cell Wnt activator in preparation of medicine for accelerating fracture healing and preventing and treating bone nonunion and non-movement or weightless bone loss
An osteocyte and activator technology, applied in drug combinations, bone diseases, pharmaceutical formulations, etc., can solve problems such as reducing bone resorption, and achieve the effects of preventing and treating weightless bone loss, improving transformation and output, important clinical significance and strategic value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
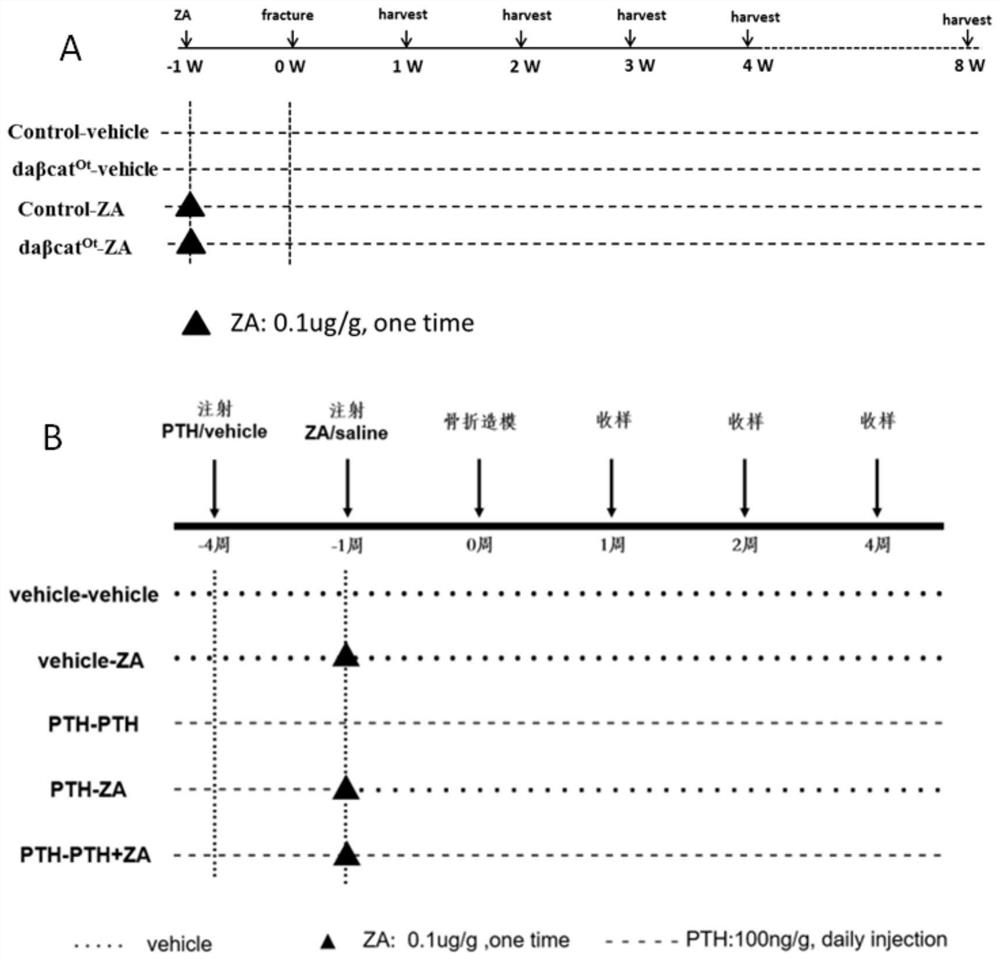
[0066] In this example, the model construction of experimental mice was carried out.
[0067] Construction of Dmp1-Cre;Catnb f / + mice.
[0068] Canonical Wnt signaling in osteocytes activates daβcat in mice Ot, is a genetically modified experimental mouse prepared by our laboratory, referring to the literature Tu, X., et al., Osteocytes mediate the anabolic actions of canonical Wnt / beta-catenin signaling in bone. Proc Natl Acad Sci U S A, 2015.112 (5 ): p. E478-86. The mouse has been established using Cre-loxP technology, and is produced by mating Catnblox(ex3)×DMP1-8kb-Cre. The Catnb gene encoding β-catenin protein is inserted into the loxp sequence at the third exon, which is cut out after being recognized by Cre recombinase. Because the 3rd exon encodes the continuous phosphorylation site sequence of β-catenin, after continuous phosphorylation, it is degraded and cleared by ubiquitination, and β-catenin without the 3rd exon will not be degraded and stabilized in the cyto...
Embodiment 2
[0073] (1) Construction of Dmp1-Cre; Catnb f / + mouse fracture model.
[0074] The fracture model was made by surgical method. Select 8-10 week old mice, 20 in each of the control group and the experimental group, and the mice are anesthetized by intraperitoneal injection of 5% chloral hydrate (0.4mg / g) at 0.08ml / 10g. Thirty minutes later, mice were operated on under continuous inhalation of buprenorphine (0.1 mg / kg). After skin preparation on the right leg of the mouse, sterilize with 70% alcohol, cut the skin parallel to the tibia, and then use a No. 11 scalpel blade to cut a 6mm incision perpendicular to the skin above the kneecap, and bluntly separate the muscle to expose the upper 1 of the tibia / 2, then make an incision perpendicular to the medial side of the patella to expose the tibial plateau, use a 0.4mm syringe needle to penetrate the bone marrow from the tibial plateau, and then use a 0.26mm needle-free pin to insert into the bone marrow cavity, and the other end t...
Embodiment 3
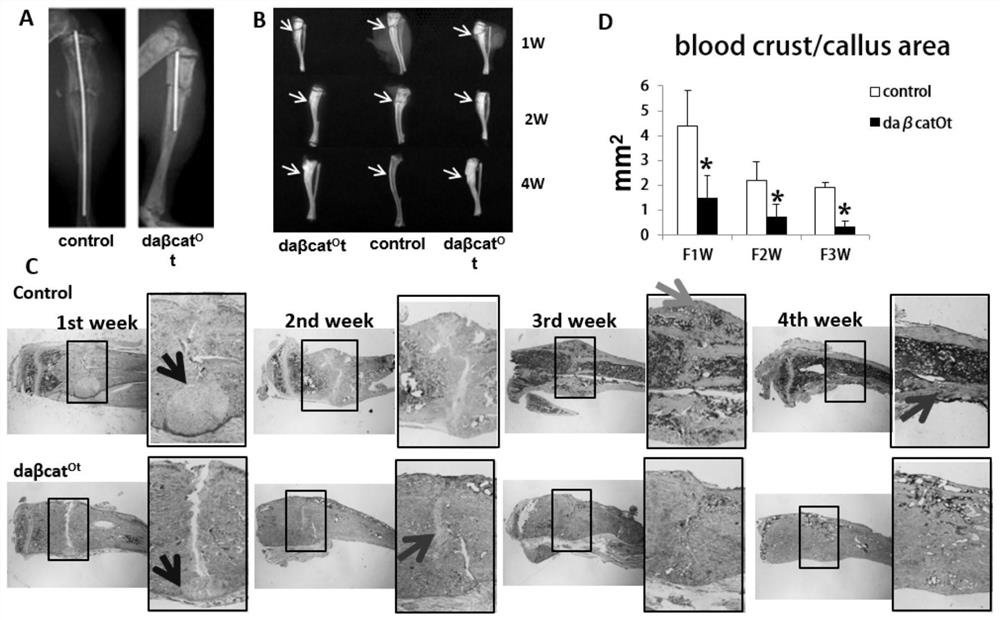
[0082] (1) Construct a mouse fracture model when bone resorption is inhibited in wild-type mice.
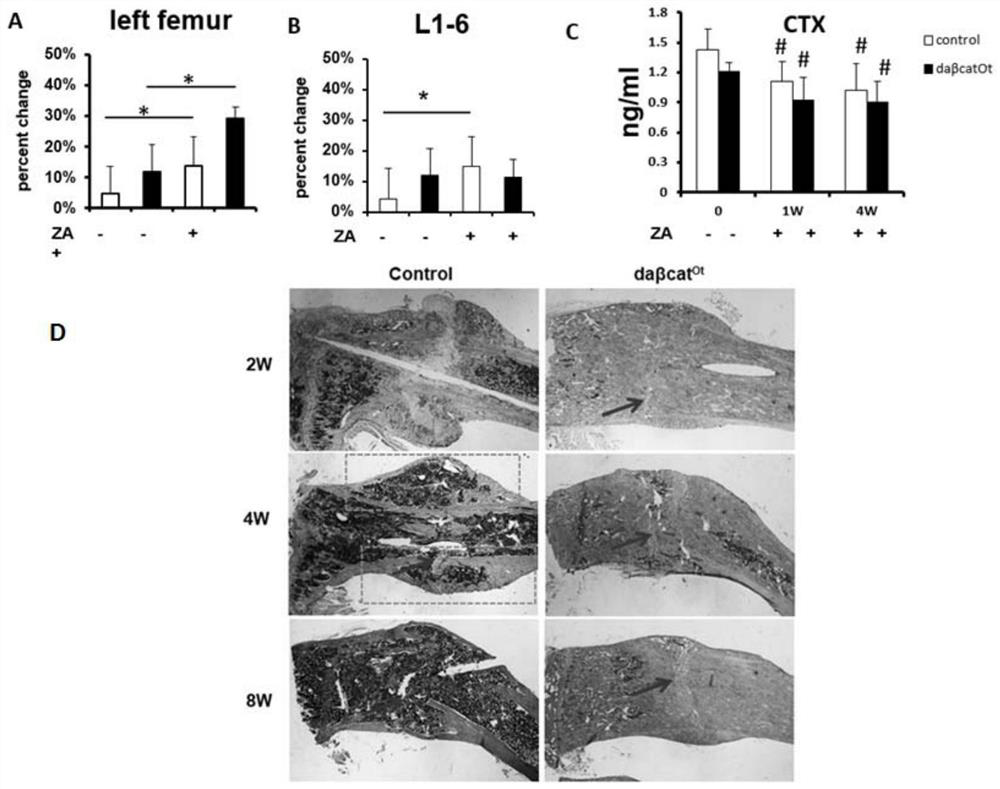
[0083] One week before the operation, 0.1 mg / kg zoledronic acid was injected intraperitoneally, and a fracture model was produced by conventional surgery. The middle part of the right tibia of the mouse was selected as the fracture point, and intramedullary fixation was performed. The specific method was as shown in Example 1. X-ray was taken every week to observe the fracture healing of the mice. (2) Construct the fracture model of wild-type mice under the condition of bone resorption inhibition and intermittent continuous injection of PTH.
[0084] Four weeks before the operation, a daily subcutaneous injection of 100ng / g PTH (bachem, product number 4011474.0005) and an intraperitoneal injection of 0.1mg / kg zoledronic acid (Novartis Pharmaceuticals) one week before the operation were performed, and the fracture model was produced by the operation method shown in Example 1. , s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com