Line eliminating method immunochromatography test paper and application thereof in CRISPR nucleic acid test
An immunochromatographic test paper and nucleic acid technology, which can be used in biochemical equipment and methods, microbial measurement/testing, and resistance to vector-borne diseases, etc., and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1. Nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography
[0054] 1. Preparation of nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography
[0055] 1. Preparation of "line elimination method" immunochromatographic test paper
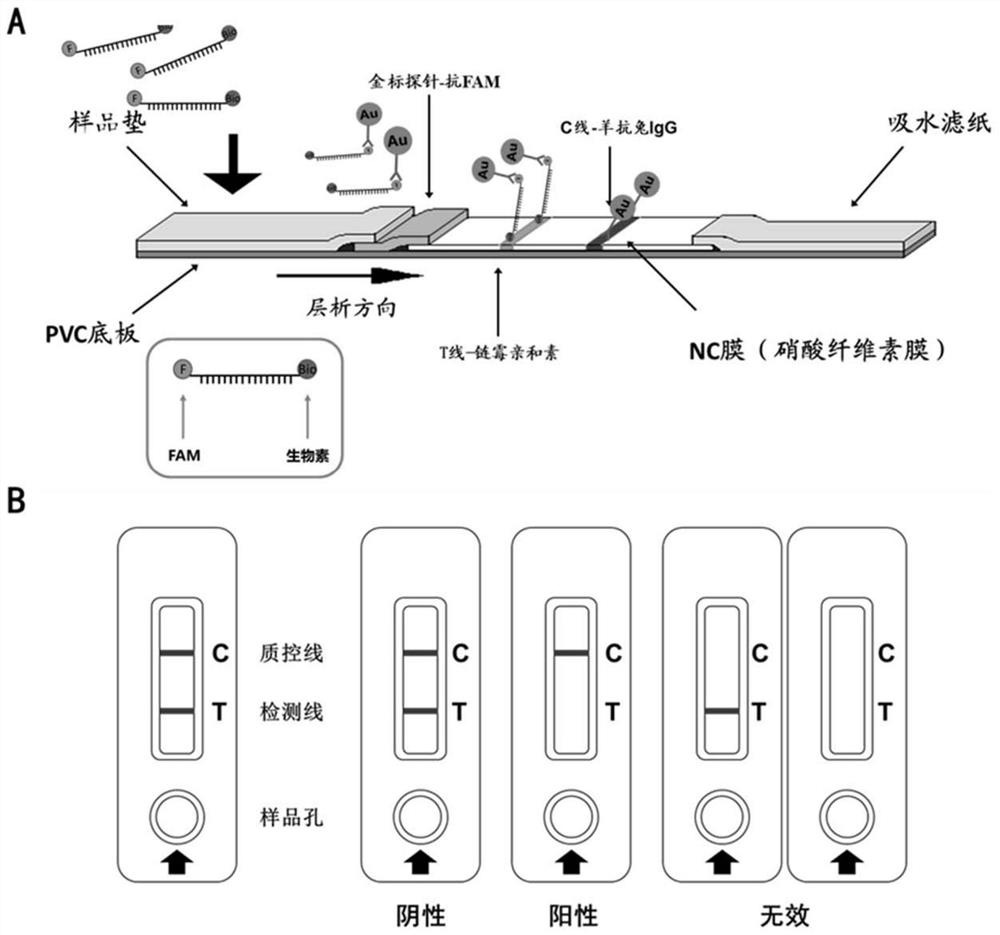
[0056] Lateral flow test strips based on the disappearing line method (such as figure 1 Shown in A) Arranged according to the flow direction, it includes the sample pad containing colloidal gold-labeled rabbit-derived anti-FITC antibody, the NC membrane containing T-line and C-line, and the absorbent filter paper.
[0057] 1), sample pad containing colloidal gold-labeled rabbit-derived anti-FITC antibody
[0058] Rabbit-derived FITC antibody was purchased from Shanghai Sangong, article number: D110003
[0059] Colloidal gold solution: add 0.4mL 10% (mass volume ratio g:mL) of chloroauric acid and 0.4mL 10% (mass volume ...
Embodiment 2
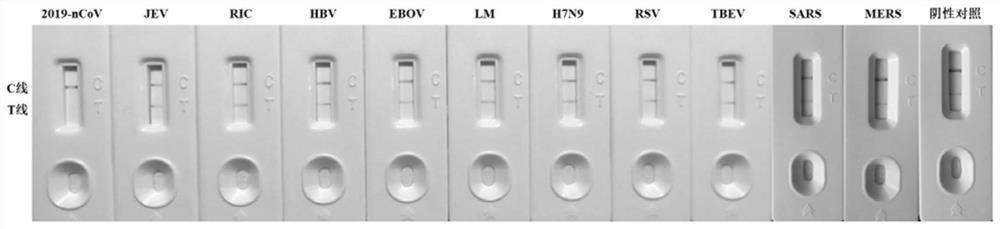
[0095] Example 2. Comparison of the nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography with the existing CRISPR lateral flow chromatography test paper in the detection of new coronavirus nucleic acid
[0096] 1. Preparation of nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography
[0097] 1. Preparation of "line elimination method" immunochromatographic test paper
[0098] Same as 1 of embodiment 1;
[0099] 2. CRISPR reaction system
[0100] 1) Obtaining reporter RNA
[0101] Same as 2 of embodiment 1;
[0102] 2) Preparation of crRNA
[0103] The crRNA consists of the LwCas13a protein binding region and the target nucleic acid target sequence binding region.
[0104] The nucleotide sequence of the crRNA is sequence 2, wherein, the 1-38th in the sequence 2 is the region bound by the LwCas13a protein, and the 39-66th is the region bound by the target nu...
Embodiment 3
[0132] Example 3. The critical concentration repeatability of the nucleic acid detection kit based on the CRISPR detection principle and the "line elimination method" immunochromatography in the detection of novel coronavirus nucleic acids
[0133] 1. Preparation of nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography
[0134] Same as the one of embodiment 2;
[0135] 2. Detection of nucleic acid detection kit based on CRISPR detection principle and "line elimination method" immunochromatography
[0136] 1), Amplification of target nucleic acid
[0137] Obtained by embodiment 2, the sensitivity of the line disappearing test paper to novel coronavirus is 10 0 copies / μL, using 10 0 The standardized novel coronavirus nucleic acid of copies / μL is used as the target nucleic acid, and a negative control group (H 2 O).
[0138] Method is the same as the second of embodiment 2.
[0139] 2) CRISPR reaction
[0140] Met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com