Fully humanized anti-hepatitis B virus monoclonal antibody as well as preparation method and application thereof
A monoclonal antibody, fully humanized technology, applied in biochemical equipment and methods, antiviral agents, chemical instruments and methods, etc., can solve the problems of blood-borne disease infection, complex quality control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
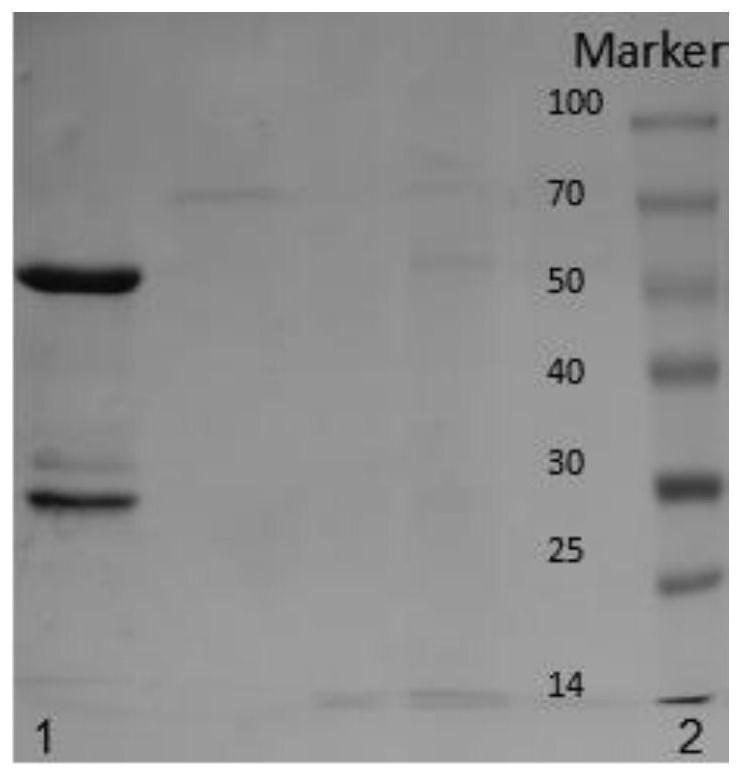
[0029] A fully humanized monoclonal antibody against hepatitis B virus, comprising a heavy chain variable region and a light chain variable region; the amino acid sequence of the heavy chain variable region is shown in SEQ ID NO.2; the light chain can be The amino acid sequence of the variable region is shown in SEQ ID NO:4.
[0030] A coding DNA of a fully humanized anti-HBV monoclonal antibody, including a heavy chain variable region and a light chain variable region; the coding DNA sequence of the heavy chain variable region is shown in SEQ ID NO.1; the The coding DNA sequence of the light chain variable region is shown in SEQ ID NO:3.
Embodiment 2
[0032] A preparation method of fully humanized anti-hepatitis B virus monoclonal antibody, comprising the steps of:
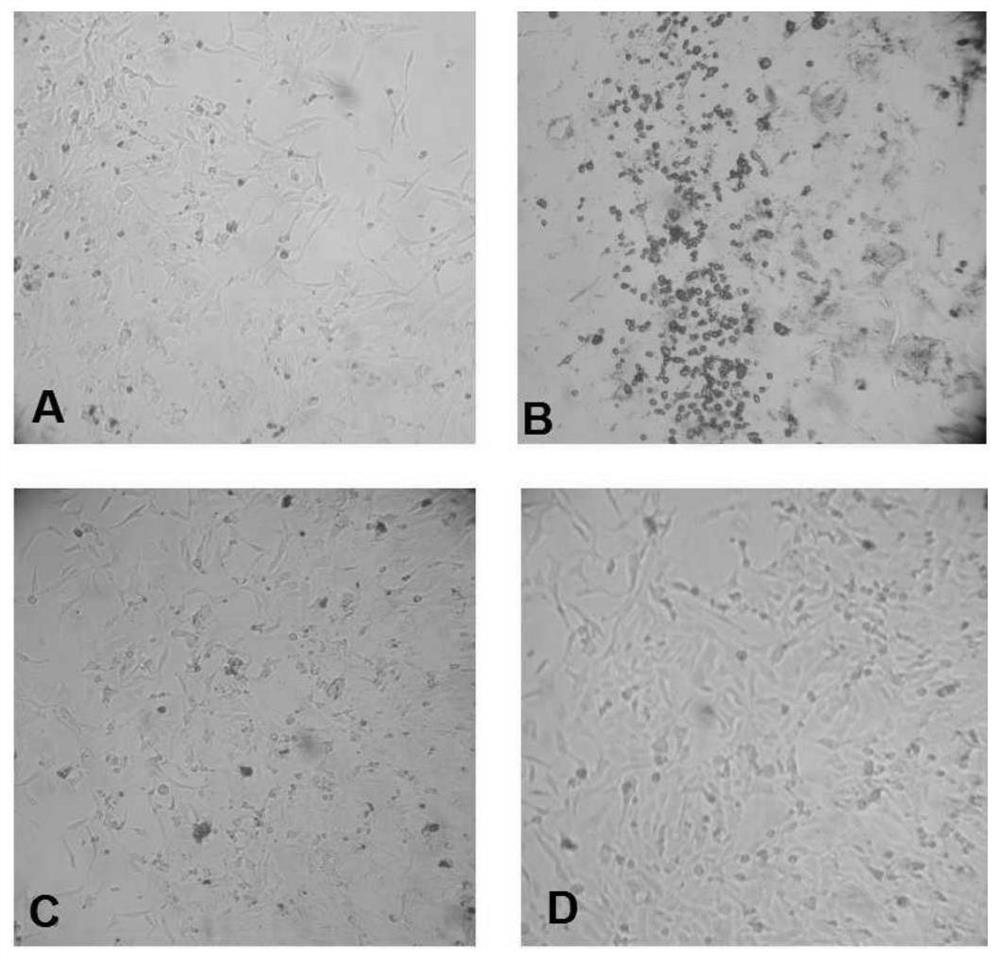
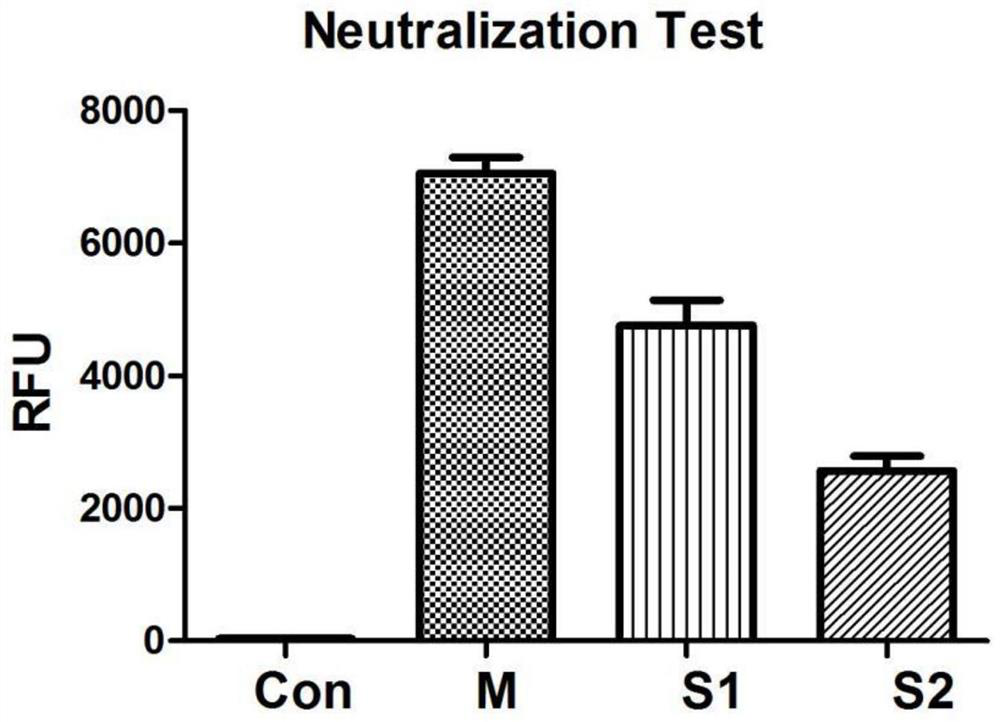
[0033] 1. Screening of positive B cells secreting HBV antibody
[0034] 1. Preparation of EBV virus
[0035] B95.8 cells were cultured in RPMI1640 medium with 10% FBS; after 96 hours, the cell culture suspension was collected and centrifuged at 400g for 10 minutes at 4°C. Harvest the supernatant, filter the supernatant with a 0.45-micron filter membrane, and obtain the EBV virus; aliquot the EBV virus and store it in a -80°C refrigerator.
[0036] 2. Isolation of peripheral blood lymphocytes
[0037] Select healthy people who have been injected with hepatitis B vaccine in the past three to six months and are negative for hepatitis B surface antigen, e antigen, and positive for hepatitis B surface antibody as B cell donors; draw 30 ml of peripheral blood from the donor, and use lymphocyte separation fluid (Histopaque -1077) were isolated from peripheral blood...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com