Preparation method of 5-aminolevulinic acid hydrochloride intermediate
A technology of aminolevulinic acid hydrochloride and intermediates, which is applied in the field of preparation of 5-aminolevulinic acid hydrochloride intermediates, can solve the problems of low purity, high price and high cost of intermediates, and achieve simple process , easy to operate, mild response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
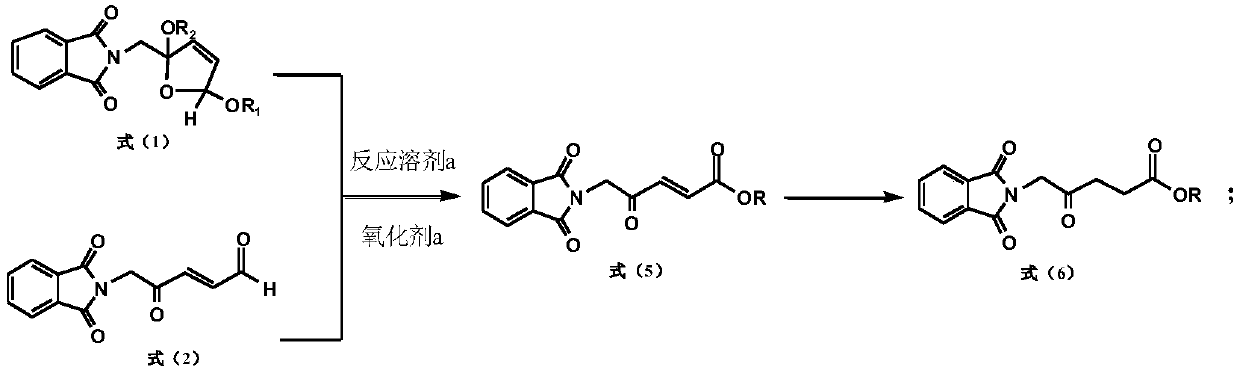
[0110] A preparation method of 5-aminolevulinic acid hydrochloride intermediate, comprising the following steps:
[0111]
[0112] Put 99.0g (0.34mol) of 2-phthalimidomethyl-2,5-dimethoxytetrahydrofuran into the reaction flask, add 700ml of acetone and stir to dissolve, then add 140ml of water; add 4g of 36% sulfuric acid and slowly Add 209g (0.34mol) potassium monopersulfate compound salt (Oxone), and stir at 20°C for 3 hours; after the reaction, filter the inorganic salt, wash the filter cake with acetone, combine the washing liquid and the filtrate to remove the solvent, add 400ml of water, and stir for half Filtrate after 1 hour, vacuum-dry after filter cake washing, obtain 5-phthalimide levulinic acid 87.0g, m.p.163 ℃, purity (HPLC, a / a%) 99.5%, yield 98.0% (with 2-phthalimidomethyl-2,5-dimethoxytetrahydrofuran).
[0113] Its proton nuclear magnetic resonance spectrum data are as follows: 'H NMR (δppm ín CDCl 3 ,400MHZ):2.71(2H,t),2.84(2H,t),4.56(2H,s),7.77-7.72(2H,m...
Embodiment 2
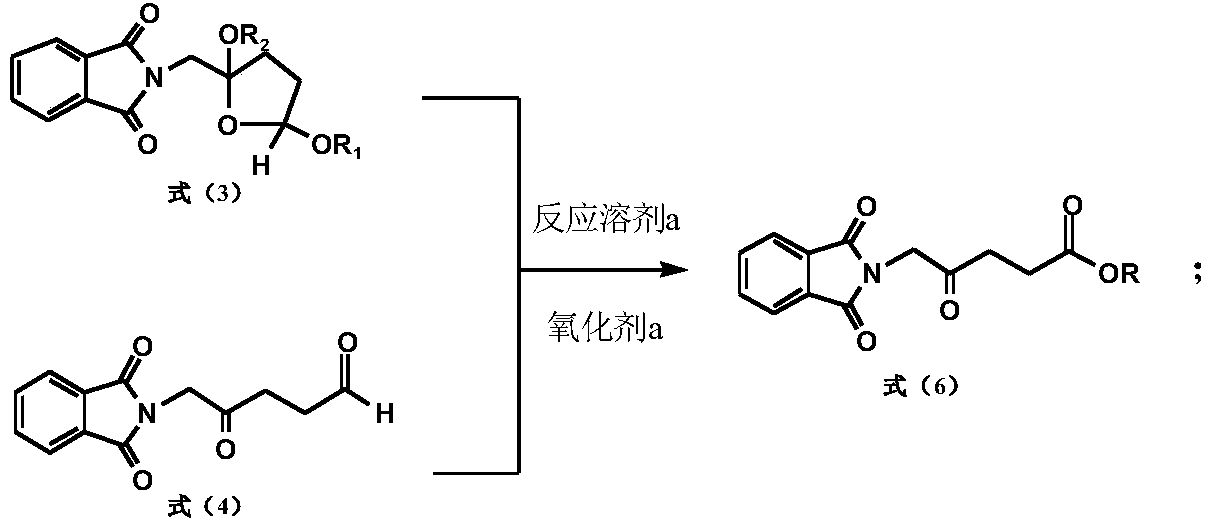
[0127] A preparation method of 5-aminolevulinic acid hydrochloride intermediate, comprising the following steps:
[0128]
[0129] Take 130.2g (0.25mol) of cis, trans-2-phthalimidomethyl-2,5-dialkoxydihydrofuran, add a catalytic amount of Raney nickel, and react with hydrogen until the hydrogen absorption is complete. Reacted for 2 hours, heated and distilled to reclaim methanol after filtering off Raney nickel, added 500ml of acetone and stirred to dissolve, then added 100ml of water; after adding 3g of 36% sulfuric acid, slowly dropped into 153.7g (0.25mol) potassium monopersulfate compound salt (Oxone), Stir at 15°C for 3 hours; after the reaction, filter the inorganic salts, wash the filter cake with acetone, combine the washing liquid and the filtrate, concentrate under reduced pressure to remove the solvent, add 300ml of water, stir for half an hour, filter, wash the filter cake, recrystallize with ethyl acetate, and vacuum dry Dry, 58.8g of 5-phthalimide levulinic ac...
Embodiment 3
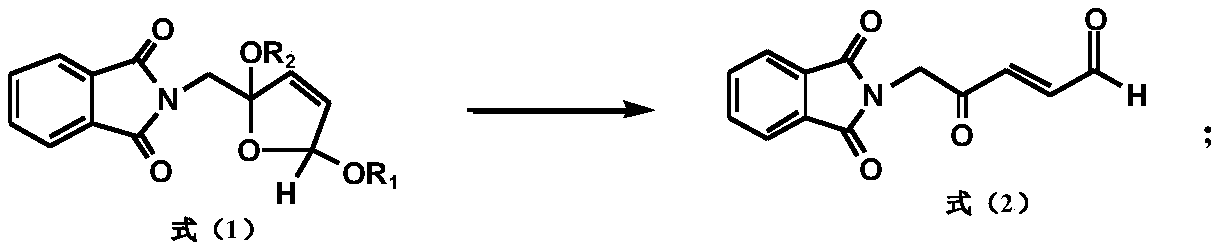
[0143] A kind of preparation method of 5-aminolevulinic acid hydrochloride intermediate, the difference with embodiment 1 is:
[0144]
[0145] Take 65.1g (0.25mol) of cis, trans-2-phthalimidomethyl-2,5-dialkoxydihydrofuran, heat and distill to remove the solvent, add 500ml of acetone and stir to dissolve, then add 100ml of water; add Slowly add 153.7g (0.25mol) of potassium monopersulfate compound salt (Oxone) after 3g of 36% sulfuric acid, and stir for 3 hours at 25°C; after the reaction, filter the inorganic salt, wash the filter cake with acetone, and combine the washing liquid and the filtrate to reduce pressure Concentrate to remove the solvent; add 1500mL of methanol and stir to dissolve, then add a catalytic amount of Raney nickel, pass hydrogen to the end of the hydrogenation reaction, filter off the Raney nickel, heat and distill to remove the solvent, add 300ml of water, stir for half an hour, filter, and wash the filter cake with water Vacuum drying, 5-phthalimi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com