Pleuromutilin derivative with amide side chain as well as preparation and application of pleuromutilin derivative
A technology for pleuromutilin and derivatives, which is applied in the fields of pleuromutilin derivatives and their preparation and application, can solve the problems of rare pleuromutilin antibiotic-resistant bacteria and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
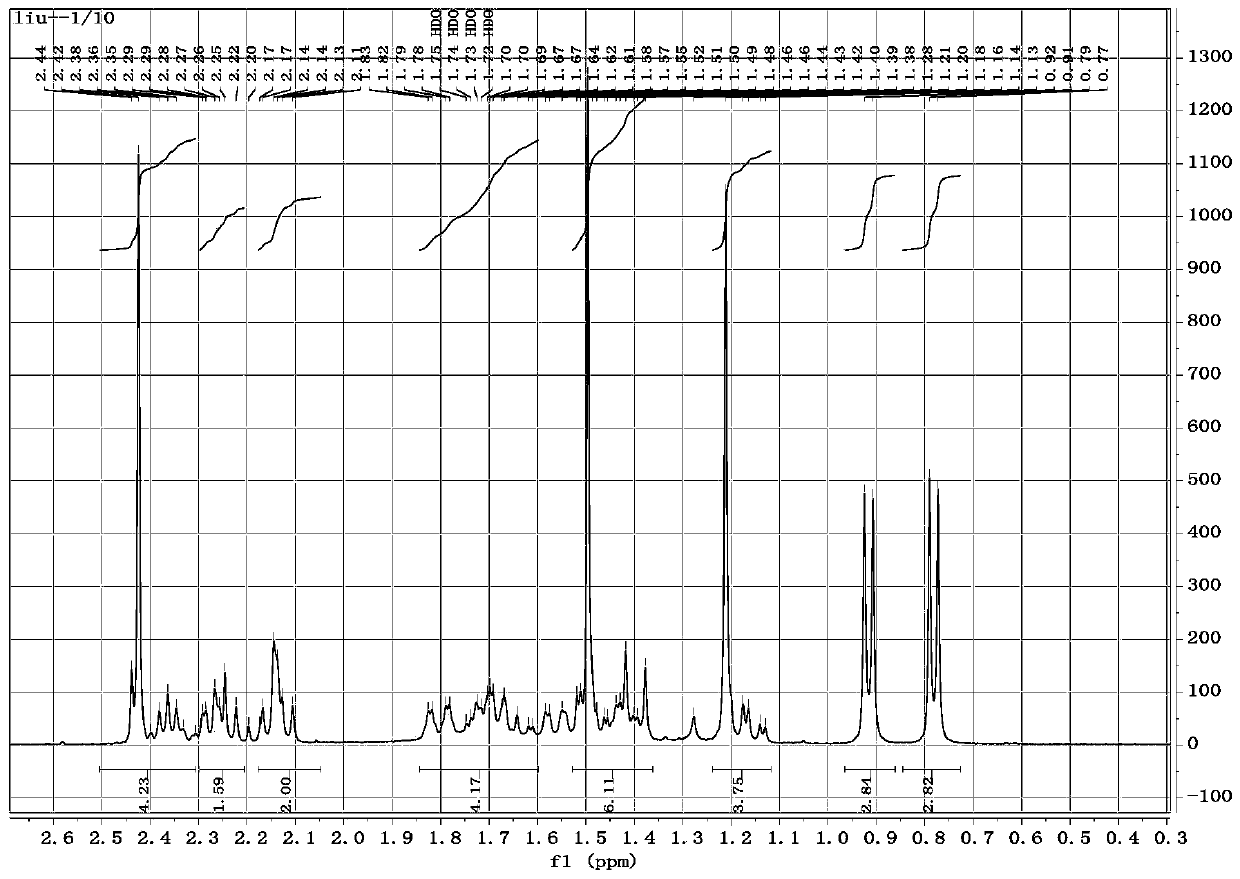
[0115](1) Preparation of Intermediate I: Dissolve 10.0g (26.5mmol) pleuromutilin in 20ml pyridine and place in an ice bath; dissolve 5.6g (29.2mmol) p-toluenesulfonyl chloride in 10ml pyridine, then slowly add For the above-mentioned pleuromutilin pyridine solution, after stirring the mixture in an ice bath for 3 h, add 50 ml each of ice water and chloroform in turn, then transfer to a separatory funnel for shaking, and let it stand until it is separated; take the organic phase, Wash with 100ml of 4mol / L sulfuric acid, 100ml of saturated sodium bicarbonate solution, and 100ml of deionized water in sequence; after washing, the organic phase evaporates the organic solution under reduced pressure, adds 20ml of isopropanol to the remaining solid, heats and dissolves, and then let cool to precipitate A large amount of white powder was suction-filtered, and the filter residue was washed with isopropanol and dried to obtain intermediate I with the structure shown in formula 3, with a ...
Embodiment 2
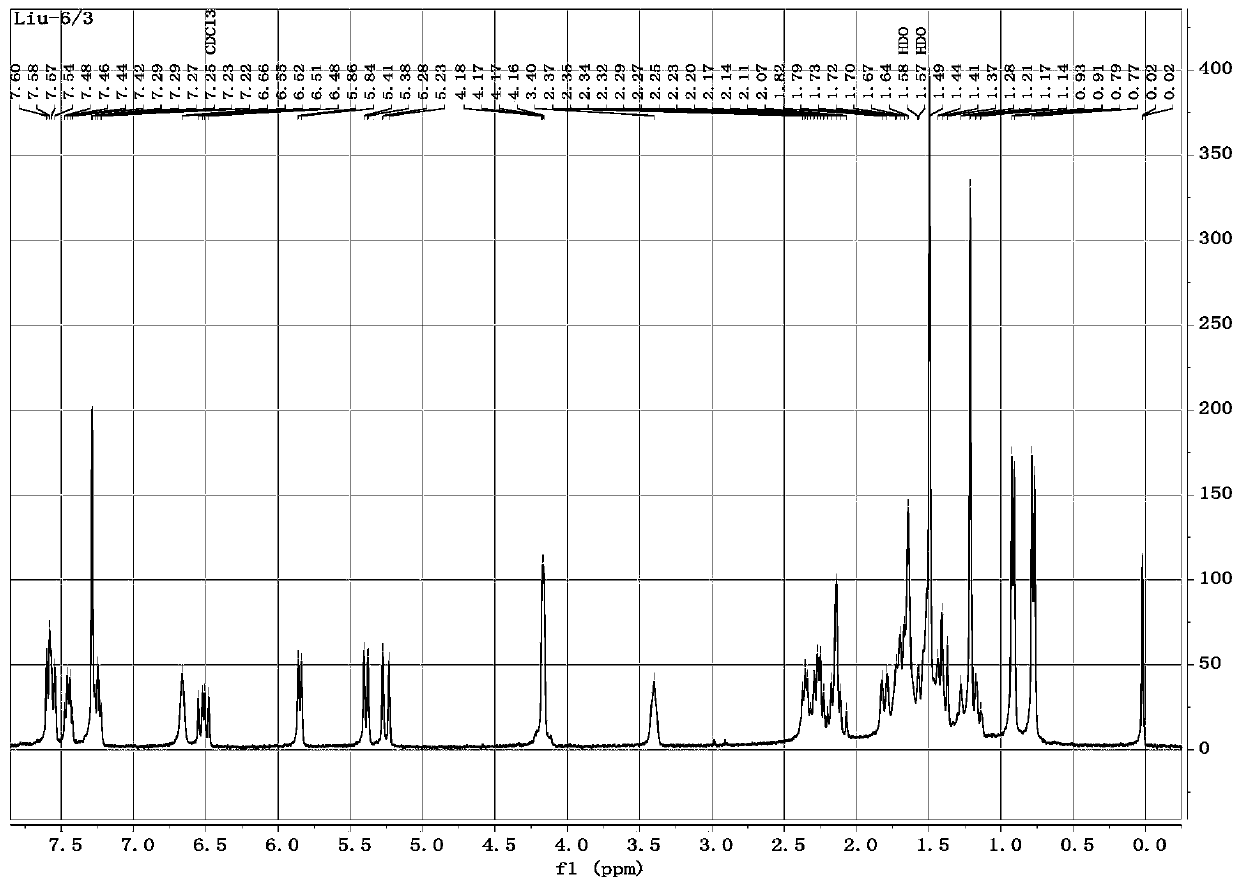
[0119] Example 2 Synthesis of 22-O-(benzamido)acetylmurein (compound 1)
[0120] Take 1g (2.65mmol) of Intermediate III and dissolve it in 23ml of dichloromethane, get 0.73g (5.30mmol) of anhydrous potassium carbonate and add it to the reaction system; dissolve 0.41g (2.92mmol) of benzoyl chloride in a small amount of dichloromethane, Slowly add dropwise to the above reaction system under ice-bath conditions, then stir the mixture in ice-bath for 3 hours, then add 50ml each of ice water and chloroform in turn, then transfer to a separatory funnel for shaking, and let it stand for layering. The organic phase was washed twice with aqueous sodium chloride solution (15% w / v) and dried over anhydrous sodium sulfate, and the organic phase was taken; the obtained organic phase was evaporated to dryness to obtain a mixture, redissolved in dichloromethane, and added with 100- 200 mesh silica gel 2g was fully mixed, and after the solvent evaporated, the above-mentioned crude product-sil...
Embodiment 3
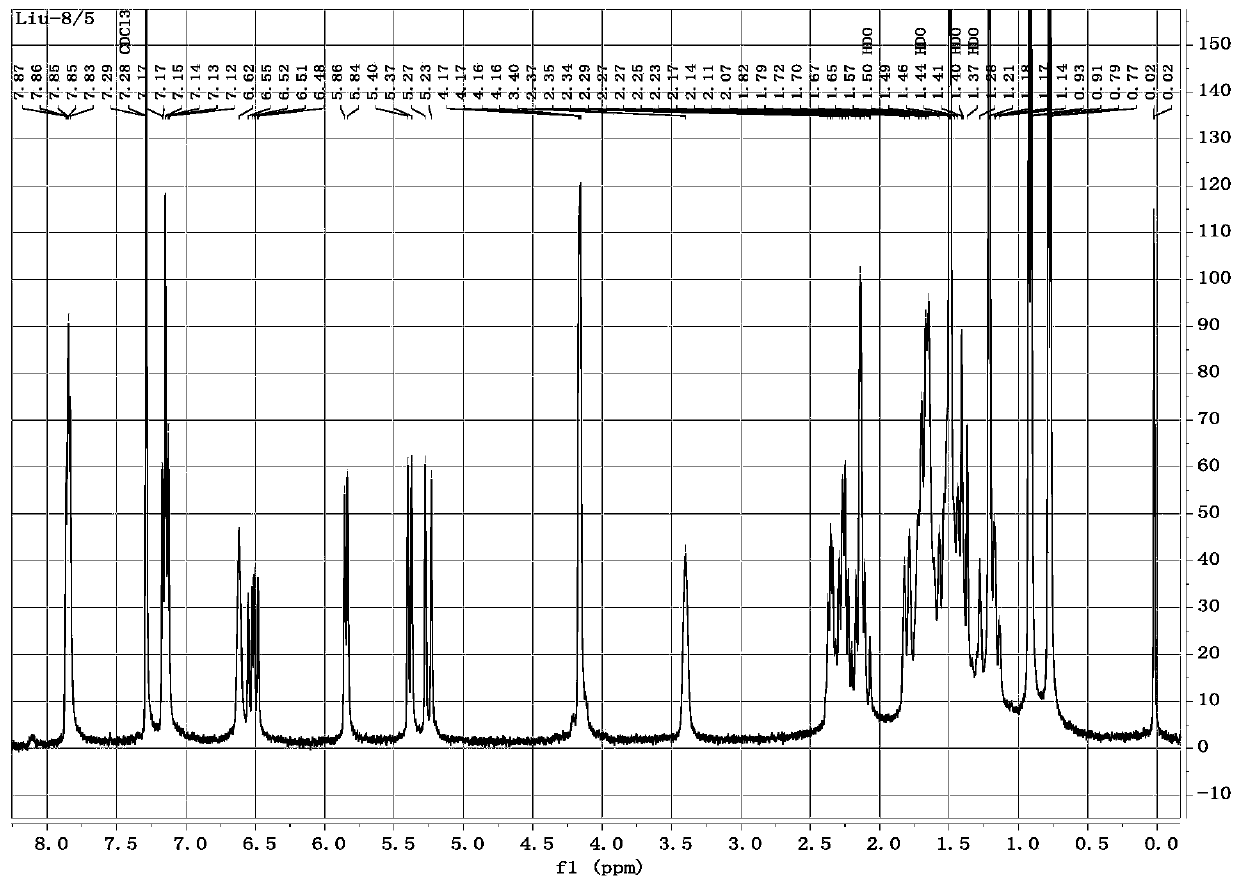
[0121] Example 3 Synthesis of 22-O-(3-methoxy-benzamido)acetyl muulin (compound 6)
[0122] Take 1g (2.65mmol) of Intermediate III and dissolve it in 23ml of dichloromethane, take 0.73g (5.30mmol) of anhydrous potassium carbonate and add it to the reaction system; dissolve 0.50g (2.92mmol) of 3-methoxy-benzoyl chloride in A small amount of dichloromethane was slowly added dropwise to the above reaction system under ice bath conditions, then the mixture was stirred in ice bath for 3 hours, then 50ml each of ice water and chloroform were added in turn, and then transferred to a separatory funnel for shaking, statically Leave it to separate layers, take its organic phase and wash twice with aqueous sodium chloride solution (15% w / v) and dry with anhydrous sodium sulfate, take the organic phase; the obtained organic phase is rotary evaporated to dryness to obtain the mixture, and the mixture is washed with dichloromethane Redissolve, add 2 g of 100-200 mesh silica gel and mix thor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com