D-D-pi-A structure photosensitive dye taking carbazole and triphenylamine as two-stage electron donors as well as preparation method and application thereof
An electron donor and photosensitizing dye technology, applied in the field of solar cells, can solve the problems of unfriendly environment, high preparation cost, hindering the application and development of metal dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1 A kind of preparation method using carbazole and triphenylamine as the photosensitizing dye of the D-D-π-A structure of two-stage electron donor
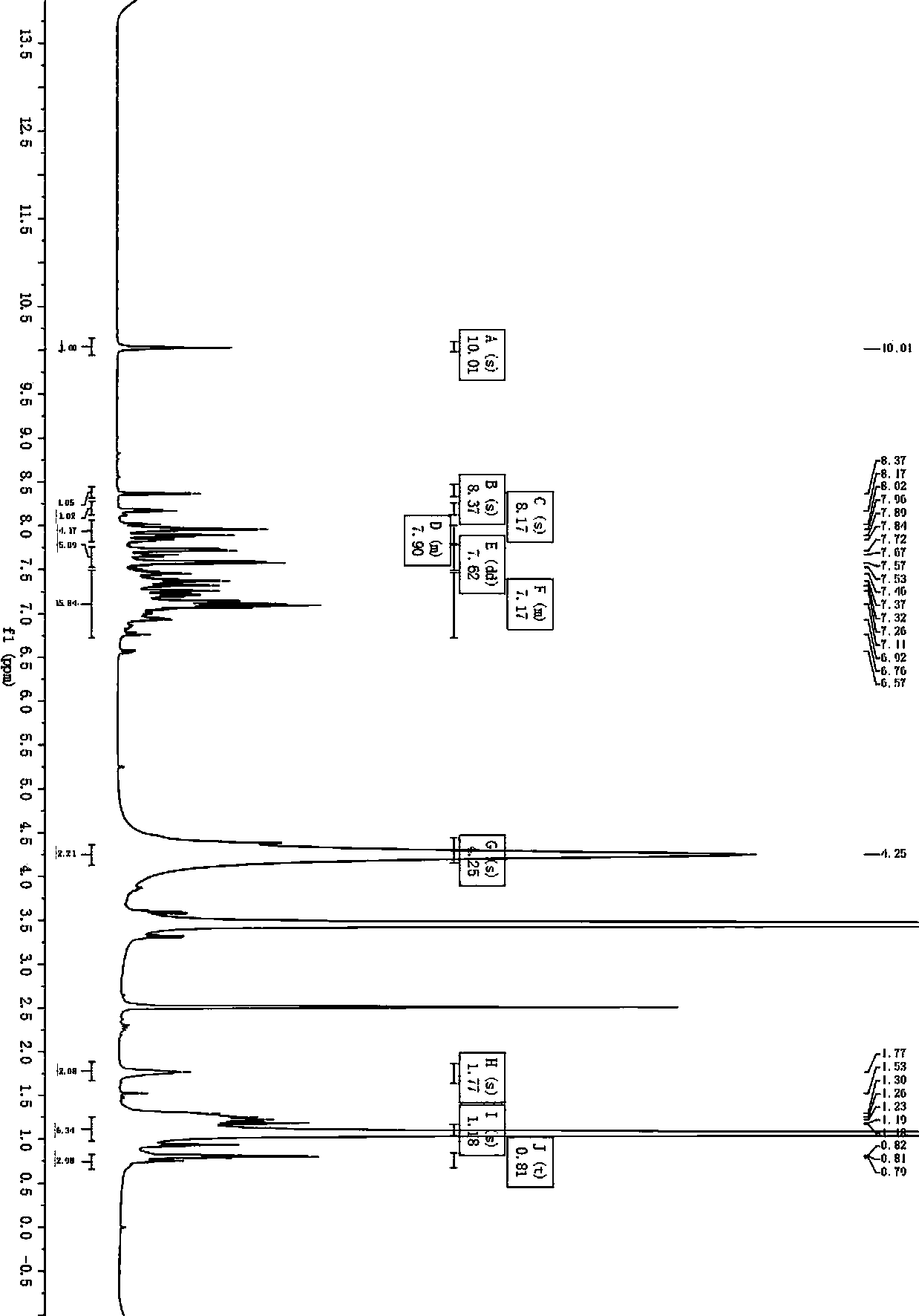
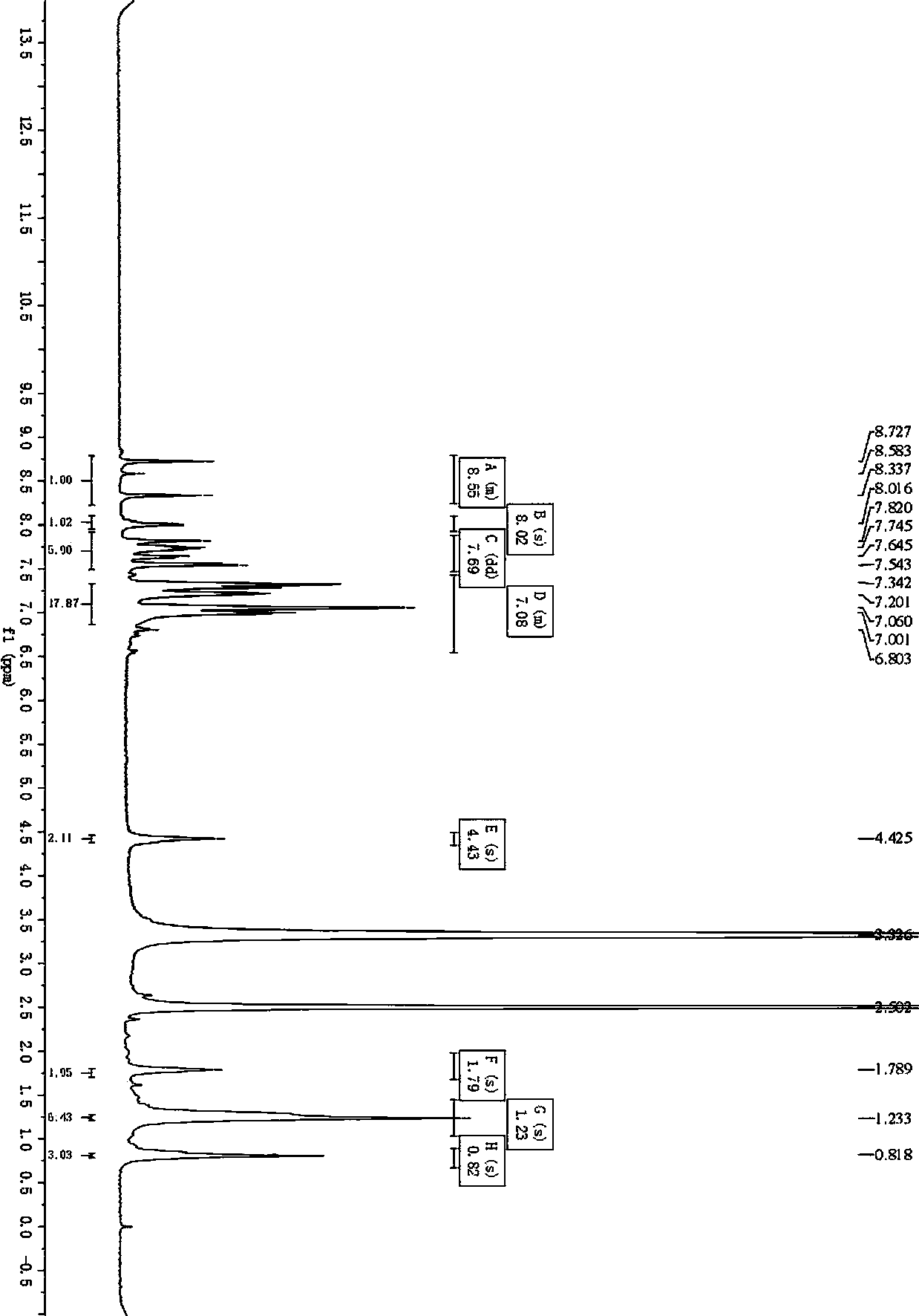
[0085] The structural formula of the photosensitizing dye with the D-D-π-A structure prepared in this embodiment with carbazole and triphenylamine as the two-stage electron donor is as follows:
[0086]
[0087] Its preparation process comprises the following steps carried out in sequence:
[0088] 1) Preparation of Intermediate I
[0089] Under nitrogen protection, add 3kg of 4-bromotriphenylamine (9.25mol) to 30L of N,N-dimethylformamide, stir and dissolve at room temperature, cool to 0°C and slowly add 7.2kg of phosphorus oxychloride dropwise (46.96mol), and the time of dropping was controlled to be 1h, and then reacted at 0°C for 1h under the protection of nitrogen, and the temperature was raised to 50°C for 10h. After monitoring the completion of the reaction, add 100L of ice water to quench, then extra...
Embodiment 2~6
[0116] Embodiment 2~6 uses carbazole and triphenylamine as the preparation method of the photosensitive dye of the D-D-π-A structure of two-stage electron donor
[0117] Examples 2 to 6 are respectively a method for preparing a photosensitive dye with a D-D-π-A structure using carbazole and triphenylamine as two-stage electron donors. Their steps are basically the same as in Example 1, except that The difference in process parameters, see Table 1 for details:
[0118] List of each process parameter in table 1 embodiment 2~6
[0119]
[0120]
[0121]
[0122]
[0123]
[0124]
Embodiment 3
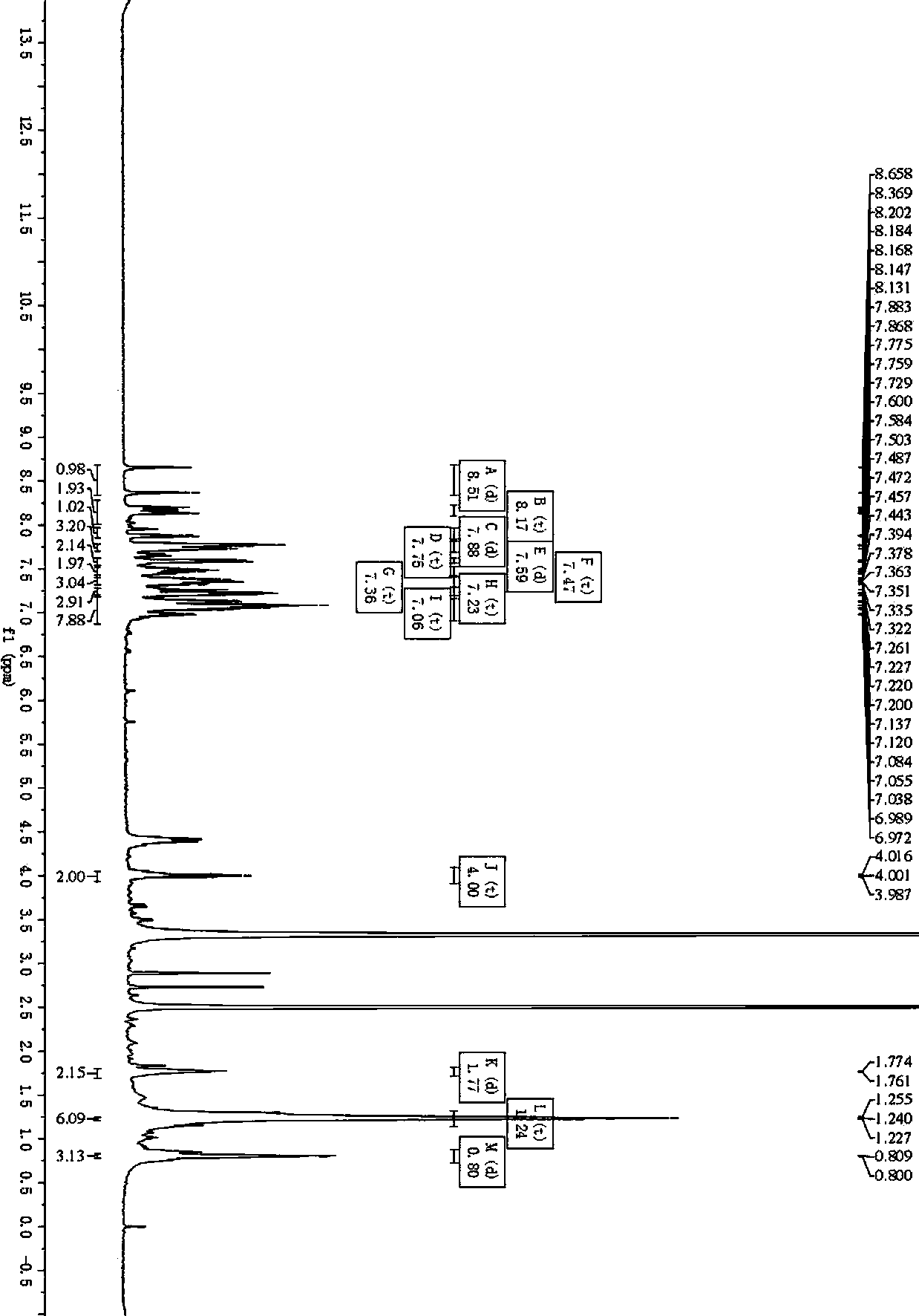
[0126] In Example 3, the proton nuclear magnetic characterization data of the prepared intermediate I are: 1 H NMR (300MHz, CDCl 3 )δ9.80(s, 1H), 7.67(d, J=9.0Hz, 2H), 7.37-7.30(m, 4H), 7.20-7.14(m, 6H), 7.01(d, J=9.0Hz, 2H ).
[0127] Intermediate I prepared in Examples 4 and 6 have the same proton nuclear magnetic data as Intermediate I prepared in Example 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com