SAM (S-Adenosyl-L-homocysteine) artificial complete antigen, preparation method and application
A cysteine and adenosine isotype technology, applied in the field of organic chemistry and immunology, can solve the problems of inability to stabilize SAH antigen and antibody, lack of SAH molecular structure antigen preparation technology, etc., and achieve high specificity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
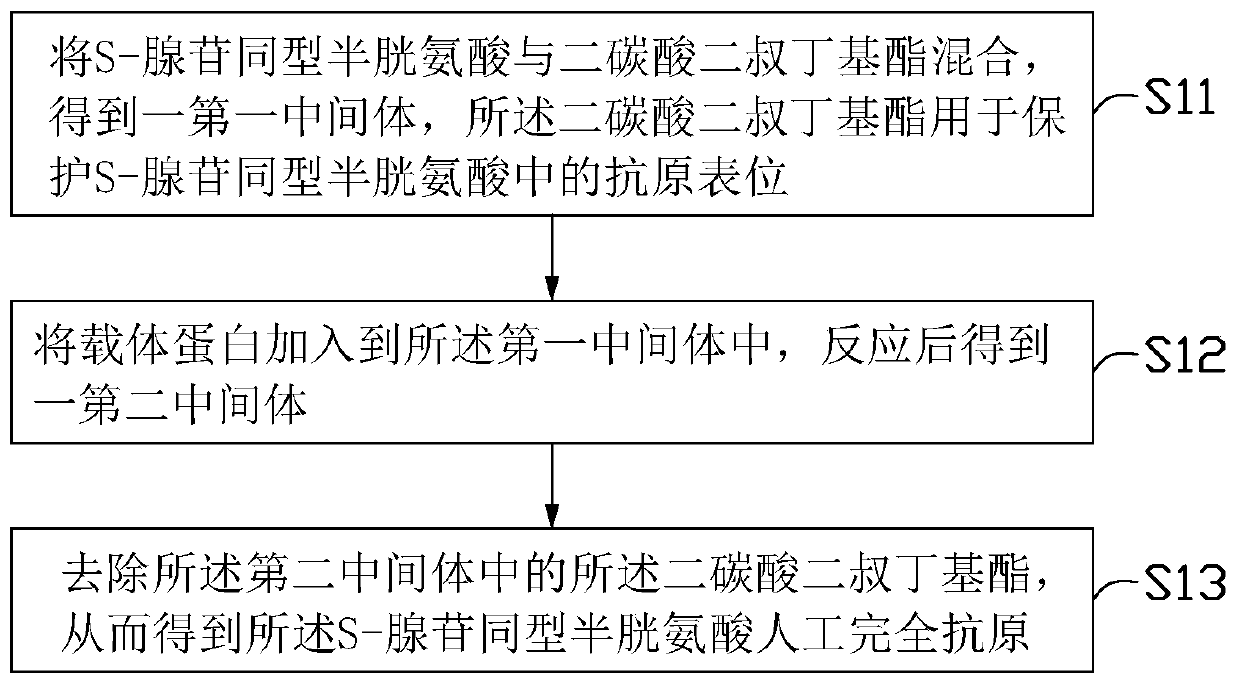
[0024] see figure 1 A preferred embodiment of the present invention provides a method for preparing an S-adenosylhomocysteine artificial complete antigen, comprising the following steps:
[0025] Step S11, mixing S-adenosyl homocysteine with di-tert-butyl dicarbonate to obtain a first intermediate, and said di-tert-butyl dicarbonate is used to protect S-adenosyl homocysteine Antigenic epitopes in acids.
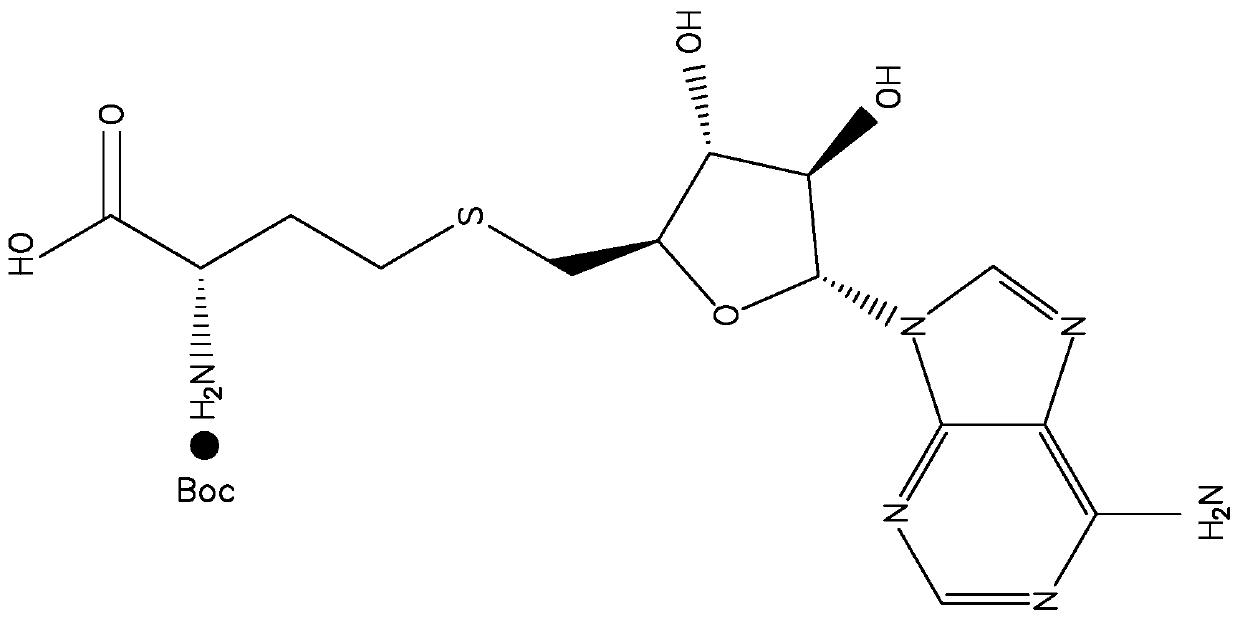
[0026] Wherein, the epitope in S-adenosyl homocysteine (SAH) is protected by di-tert-butyl dicarbonate (Boc). Such as figure 2 As shown, the SAH molecule is a combination of homocysteine (Hcy) molecule and adenosine through the -S-C- bond, and the only carboxyl group in the molecule is located at the homocysteine part, and together with its adjacent α-amino group, it forms a characteristic Characterizing said antigenic epitopes. During the subsequent preparation of the antigen, the α-amino group must not react. Therefore, the α-amino group of the cysteine pa...
Embodiment 1
[0044] Step 1: Dissolve 8.5mg of sodium bicarbonate in 2mL of deionized water, add 19.25mg of SAH after complete dissolution, and then slowly add tetrahydrofuran:dioxane as an amphiphilic solubilizer at a ratio of 1:1 in an ice-bath environment After the dropwise addition, stir magnetically at 0°C for 15 minutes to observe the dissolution of SAH, then add 2mL of tetrahydrofuran solution dissolved with 11.5 μL di-tert-butyl dicarbonate and continue the reaction at 0°C for 40 minutes, then raise the temperature to 25 ℃ to continue the reaction in the dark for 18 hours. After the reaction, add 5 mL of ethyl acetate to extract three times, separate the water phase and freeze it at -20°C, and recover the crystals by freeze-drying with a lyophilizer. The product is the first intermediate (namely Boc-SAH). Save for later use.
[0045] In the second step, 10 mg of the first Boc-SAH intermediate prepared in the first step and 30 mg of BSA were dissolved in 3 mL of PBS solution with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com