Application of bromodomain protein 4 (BRD4) inhibitor JQ1 or derivative thereof in preparation of medicine for treating sepsis intestinal barrier injury
A barrier damage and inhibitor technology, applied in the field of medicine, can solve problems such as unreported application of JQ1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
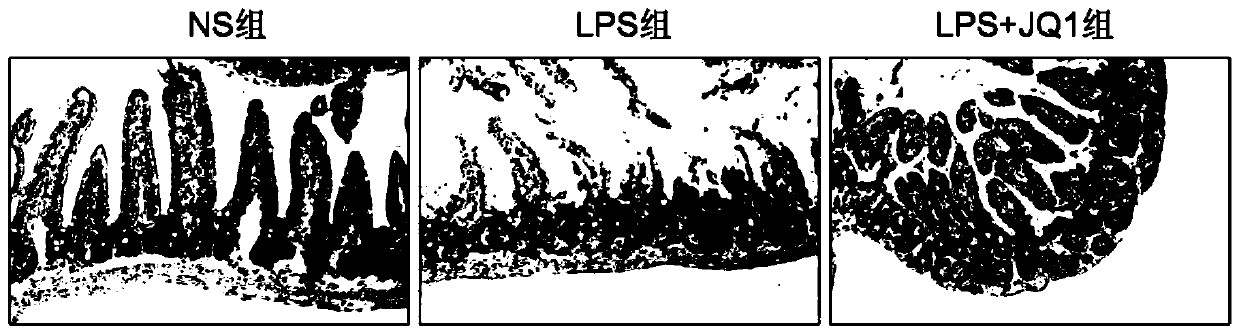
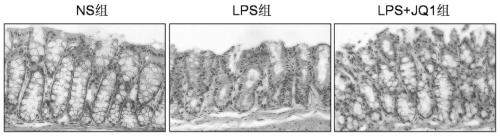
[0035] The animals selected for this experiment were 8-week-old C57BL / 6 male mice, weighing about 25 grams, purchased from Hunan Slack Jingda, license number: SCXK (Xiang) 2019-0004, and the mice were placed in an artificial day and night cycle lighting environment for 12 hours Raised in the middle, free access to food and water. C57BL / 6 male mice were randomly divided into normal saline control group (5 mice), LPS modeling group (10mg / kg, 5 mice), JQ1 administration group (50mg / kg, 5 mice). Injection of corresponding doses of normal saline or JQ1 drugs, intraperitoneal injection of 10mg / kg LPS 1h later, 24h later (starting timing after LPS injection), anesthetized mice with 4% chloral hydrate intraperitoneal injection, after complete anesthesia, intestinal tissue was taken and placed in Fix in 4% paraformaldehyde overnight for tissue HE staining.
[0036] Intestinal tissue HE staining steps:
[0037] (1) The fixed intestinal tissues were dehydrated with graded alcohol, embe...
Embodiment 2
[0045] The source of experimental animals, feeding conditions and administration process are all referred to Example 1. After the mice were anesthetized, the intestinal tissues were taken and stored at -80°C for fluorescent quantitative PCR detection.
[0046] Intestinal tissue fluorescent quantitative PCR steps:
[0047] (1) RNA extraction and reverse transcription: Homogenize the tissue with 1ml Trizol lysate, add 200μl chloroform, mix well and let stand for 5min; centrifuge at 12000g for 15min at 4°C, transfer the upper aqueous phase, add an equal volume of isopropanol and mix well centrifuge at 12000g for 10min at 4°C; discard the supernatant and add 1ml of 75% ethanol; centrifuge at 7500g for 5min at 4°C and discard the supernatant; add 20 μl of enzyme-free water to dissolve after drying; : K1621) instructions for reverse transcription of RNA into cDNA.
[0048] (2) RT-PCR: 10 μl reaction system: 5 μl TB reaction solution, 0.2 μl ROX dye, 0.4 μl each of upstream and dow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com