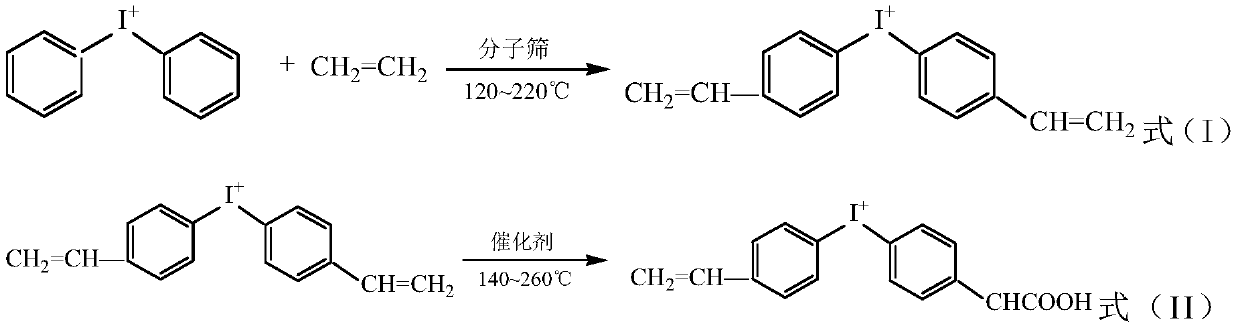
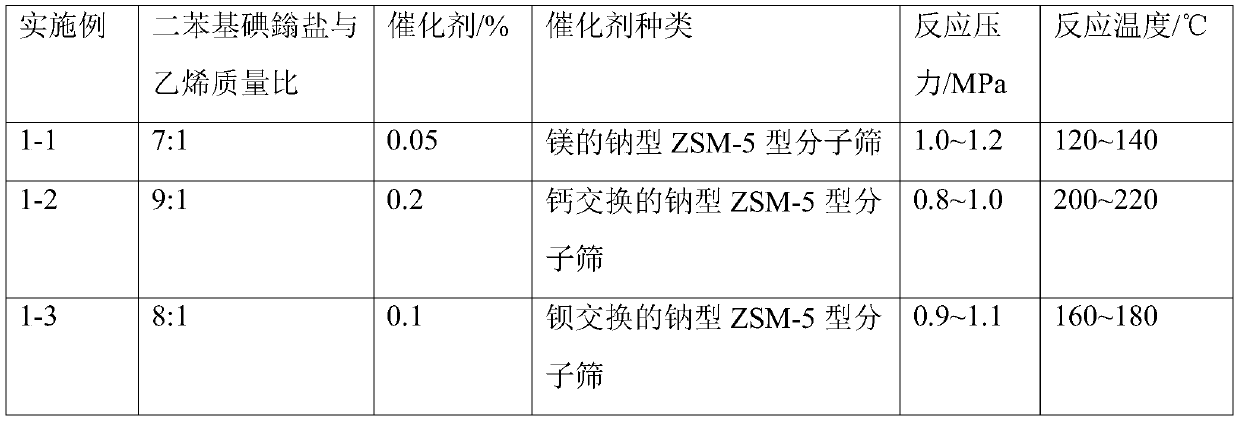
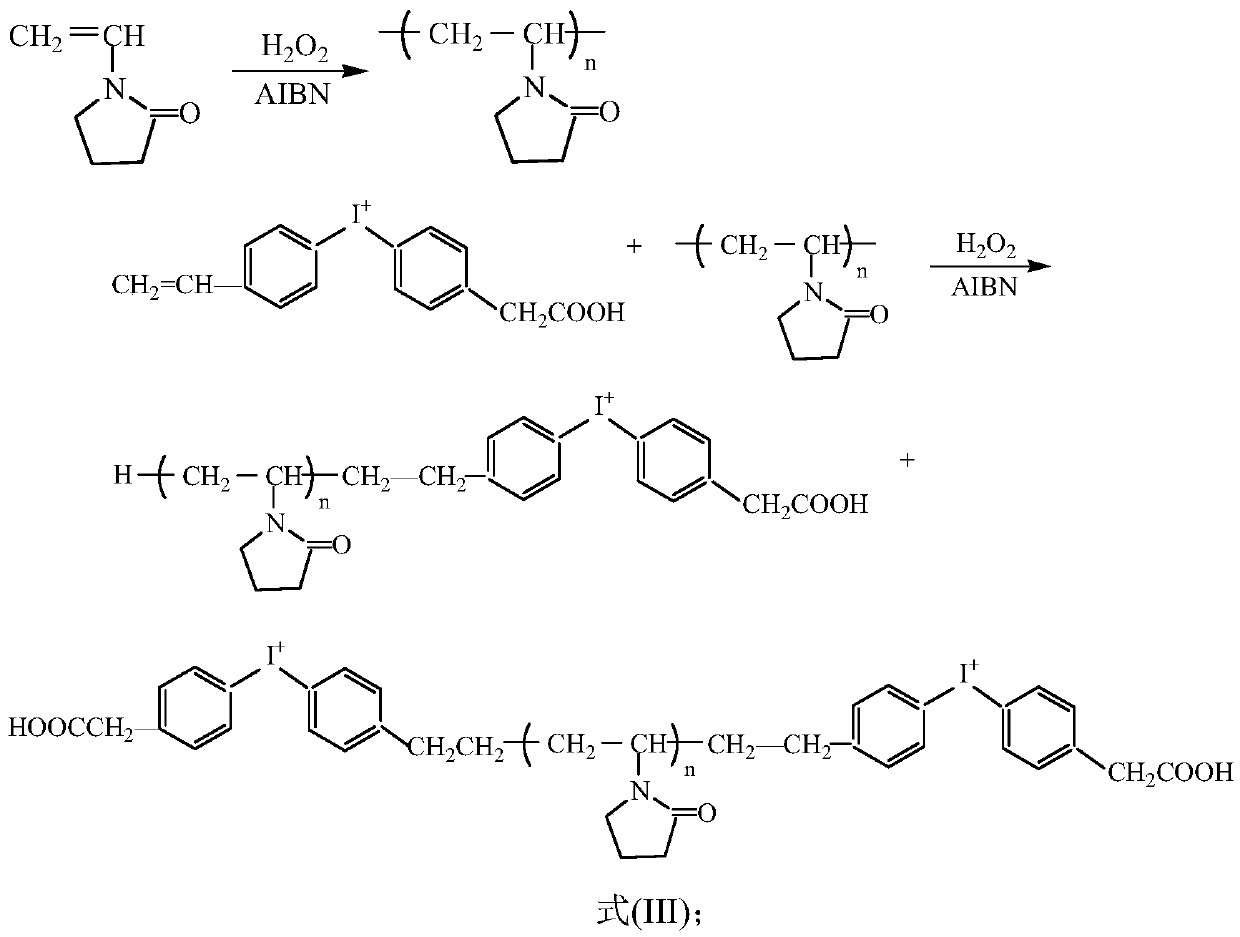
Preparation method of cationic surfactant and application thereof
A surfactant and cation technology, applied in the direction of cationic surface active compounds, surface active detergent compositions, polymer surface active compounds, etc. Modified cellulose and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-1
[0032] 1. Synthesis of (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone
[0033] The monomer vinylpyrrolidone (NVP) is configured into an aqueous solution with a mass fraction of 45%, using a small amount of hydrogen peroxide (0.2% of NVP) as a catalyst, under the action of azobisisobutyronitrile (0.3% of NVP), in Initiate polymerization at 40-50°C, react for 2 hours, and the monomer polymerization conversion rate is 93-95%. Add the (4-vinylphenyl-4'-methylenecarboxyphenyl) iodonium (0.5% NVP) prepared in Example 1-1, react for 0.2 hours, and cap the polymer, (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone is obtained.
[0034] Then add 0.2% ammonia water to the polymer to decompose the remaining azobisisobutyronitrile. The polymer is spray-dried under hot air at 120-135°C to obtain powdery (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone. (4-vinylphenyl-4'-methylenecarboxy...
Embodiment 2-2
[0045] 1. Synthesis of (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone
[0046] The monomer vinylpyrrolidone (NVP) was configured into an aqueous solution with a mass fraction of 50%. Use a small amount of hydrogen peroxide (0.3% NVP) as a catalyst, under the action of azobisisobutyronitrile (0.4% NVP), initiate polymerization at 50-60°C, react for 3 hours, and the monomer polymerization conversion rate is 96~98%.Add (4-vinylphenyl-4-methylenecarboxyphenyl) iodonium (consumption is 0.8% NVP) that embodiment 1-1 makes, react 0.5 hour, to polymer Capping is performed to obtain (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-blocked polyvinylpyrrolidone.
[0047] Add 0.3% ammonia water to the polymer to decompose the remaining azobisisobutyronitrile. The polymer is spray-dried under hot air at 135-150°C to obtain powdery (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone. (4-vinylphenyl-4'-methylenecarboxyphenyl)...
Embodiment 2-3
[0053] 1. Synthesis of (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone
[0054] The monomer vinylpyrrolidone (NVP) was configured into an aqueous solution with a mass fraction of 55%. Use a small amount of hydrogen peroxide (0.4% NVP) as a catalyst, under the action of azobisisobutyronitrile (0.5% NVP), initiate polymerization at 60-70 ° C, react for 4 hours, the monomer polymerization conversion rate is 97 ~99%. Add the (4-vinylphenyl-4-methylenecarboxyphenyl) iodonium (amount of 1% NVP) obtained in Example 1-2, react for 0.6 hours, and cap the polymer to obtain (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone.
[0055] Add 0.4% ammonia water to the polymer to decompose the remaining azobisisobutyronitrile. The polymer is spray-dried under hot air at 150-165°C to obtain powdery (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone. (4-vinylphenyl-4'-methylenecarboxyphenyl) iodon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com