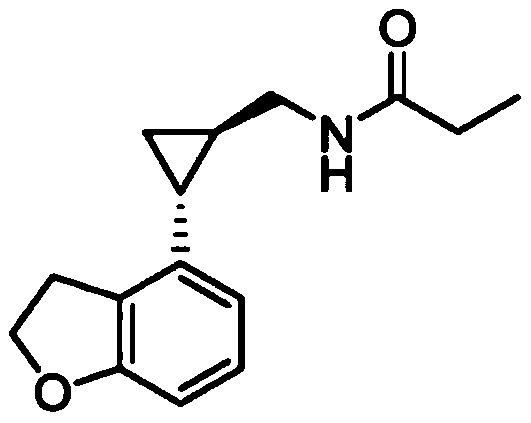
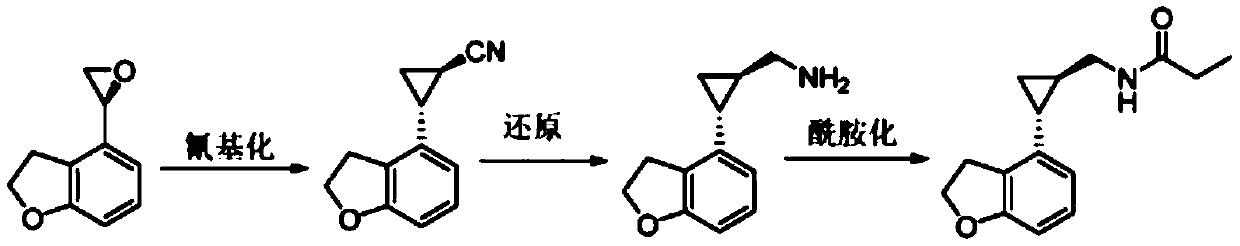
Preparation method of tasimelteon key intermediate
A technology of tasimelteon and intermediates, which is applied in the field of preparation of key intermediates of tasimelteon, can solve problems such as being unsuitable for large-scale production, high toxicity, etc., and achieves safe and controllable production process, low cost and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1. metal catalyst: the preparation of the Ru catalyst of salen ligand
[0036] At 25-30°C, 80g RuCl 3 The hydrate was dissolved in 4L of ethanol and stirred to dissolve. Subsequently, 342.8 g of 4-isopropyltoluene was added to the reaction mass. The reaction mass was heated to 83-85°C for about 12h. Stop heating, cool to 0-5 ° C and stir for 2-3h. After filtering and eluting with 80 ml of ethanol, the eluate was collected and dried in vacuo to obtain 61 g of solid. Detected as RuCl by H NMR 2 (p-cymene) dimer.
[0037] In inert gas N 2 Under the protection of , the temperature is 0°C, add 260ml of tetrahydrofuran solution (3mmol) of lithium diisopropylamide to 2L salen ligand tetrahydrofuran solution (1.5mmol), after the addition is completed, stir at 25-30°C for 1h, then, Add RuCl dropwise at 0°C 2 (p-cymene) dimer (61 g, 0.75 mmol) and 132 ml of pyridine, the reaction mass was stirred overnight at 25-30° C. to obtain a dark red solution. The dark r...
Embodiment 2
[0038] Example 2. Preparation of ethyl (1R, 2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropane-1-carboxylate
[0039] Dissolve 106g of 4-vinyl-2,3-dihydrobenzofuran in 1060ml of ethyl acetate, add 11.9g of the Ru catalyst with salen ligand prepared in Example 1 under stirring conditions, and heat to 50°C , at N 2Under protection, a solution of ethyl diazoacetate in ethyl acetate (126.4 g of ethyl diazoacetate dissolved in 1060 ml of ethyl acetate) was added dropwise within 30 min, and stirred for another 16 h. Qualitatively detected by HPLC, the reaction has been completed. Wash twice with 1 L of water at 10-20 °C, combine the aqueous layers, and extract with 500 ml of ethyl acetate. After extraction, the organic layers were combined and concentrated. The concentrated organic layer was purified by silica gel chromatography, and gradient elution was performed with 2 L of petroleum ether and 5 L of a mixture of ethyl acetate and petroleum ether at a volume ratio of 1:9. The elua...
Embodiment 3
[0040] Example 3. Preparation of (1R,2R)-2-(2,3-dihydro-4-benzofuryl)cyclopropanecarboxylic acid
[0041] Dissolve 148g of (1R, 2R)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropane-1-carboxylic acid ethyl ester prepared in Example 2 in 268ml of tetrahydrofuran and 134ml of ethanol mixed solution At a temperature of 10-20° C., NaOH solution (46.21 g NaOH dissolved in 402 ml water) was added thereto. The reaction mass was heated to 40 °C for 16 h. Concentrate without residues of tetrahydrofuran and ethanol solvents. The mixture was dissolved in 300 ml of water and washed twice with 500 ml of dichloromethane. At 10-20°C, add 85% H 3 PO 4 Aqueous solution The aqueous layer was acidified to pH=3. The acidified aqueous layer was extracted twice with 500 ml of ethyl acetate and concentrated to obtain 119 g of crude solid. As detected by HPLC, the chiral purity is 92.2%, the enantiomer is 3.3%, and the diastereomer is 4.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com