Construction method of characteristic map of Yizhiren formula granules and its quality detection method
A technology of formula granules and characteristic map, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of lack of quality standard Yizhiren formula particles, unstable volatile oil properties, and few Yizhiren formula particles, etc. Monitoring, simple method, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Preparation of reference substance solution: take the reference substance of grapefruit ketone and kaempferol, dissolve with solvent to obtain reference substance solution;
[0053] Preparation of the test solution: take Yizhiren formula granules, add an extraction solvent for extraction, filter the extract, and take the subsequent filtrate to obtain the test solution;
[0054] The reference substance solution and the test solution are injected into an ultra-high performance liquid chromatograph for detection. The mobile phase A used by the ultra-high performance liquid chromatograph is acetonitrile, and the mobile phase B is a volume fraction of 0.05% to 0.15%. Formic acid aqueous solution, elution mode is gradient elution.
[0055] In one specific embodiment, the gradient elution includes the following procedures:
[0056] 0 ~ 10min, the volume percentage of mobile phase A increased from 8% to 16%;
[0057] 10-25min, the volume percentage of mobile phase A increased...
Embodiment 1
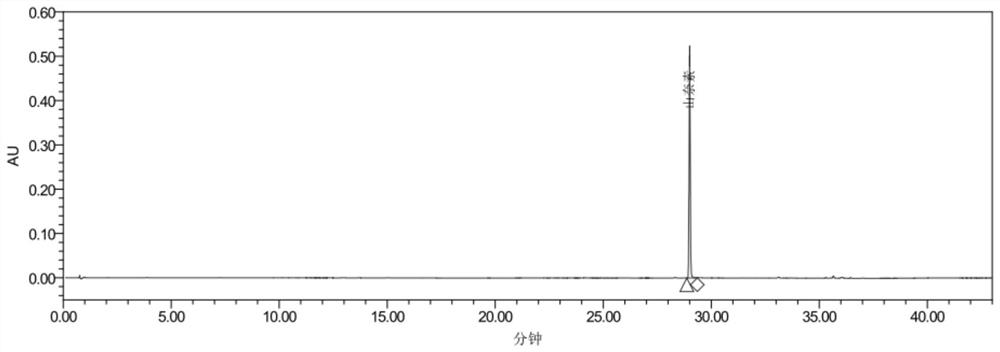
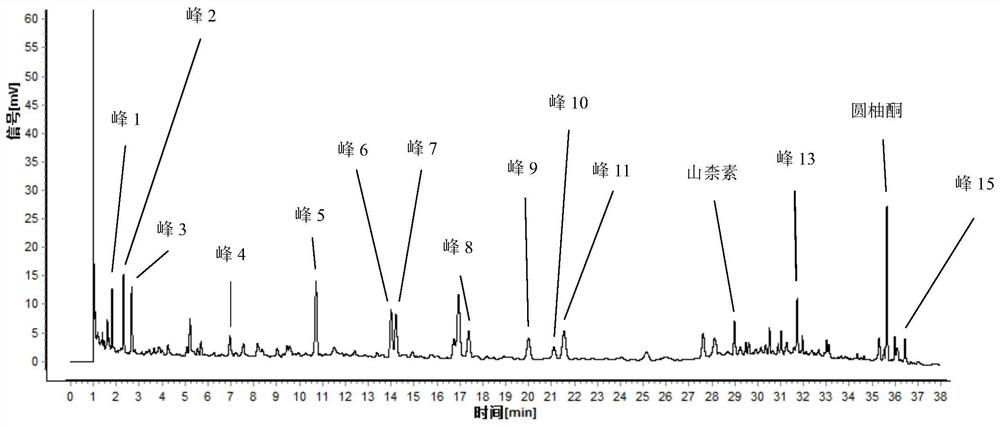
[0089] This embodiment is a method for constructing a characteristic map of Yizhiren formula granules.
[0090] 1. Chromatographic conditions and system applicability experiment: Chromatographic column: Agilent ZORBAX SB-C18 column, 2.1mm×100mm, 1.8um; acetonitrile is used as mobile phase A, and 0.1 mL of formic acid solution per 100 mL of water is used as mobile phase B , the gradient elution is shown in Table 2; the column temperature is 30°C, the flow rate is 0.3ml / min, the detection wavelength is 254nm, and the number of theoretical plates should not be less than 100,000 calculated according to the yuzuone peak.
[0091] Table 2
[0092] time (minutes) Mobile phase A (%) Mobile phase B (%) 0~10 8~16 92~84 10~25 16~23 84~77 25~35 23~73 77~27 35~38 73~8 27~92
[0093] 2. Preparation of the solution
[0094] Preparation of reference substance solution: take appropriate amount of naringenone and kaempferol reference substance, accura...
Embodiment 2
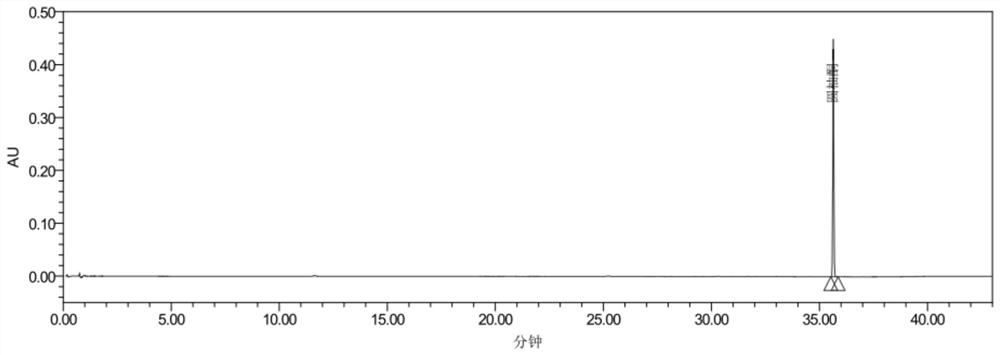
[0118] The present embodiment is a method for determining the content of yuzuone in Yizhiren formula granules.
[0119] 1. Chromatographic conditions and system applicability experiment: Chromatographic column: Agilent ZORBAX SB-C18 column, 2.1mm×100mm, 1.8um; acetonitrile is used as mobile phase A, and 0.1 mL of formic acid solution per 100 mL of water is used as mobile phase B , the gradient elution is shown in Table 5; the column temperature is 30°C, the flow rate is 0.3ml / min, the detection wavelength is 254nm, and the number of theoretical plates should not be less than 100,000 calculated according to the juvenone peak.
[0120] table 5
[0121] time (minutes) Mobile phase A (%) Mobile phase B (%) 0~10 8~16 92~84 10~25 16~23 84~77 25~35 23~73 77~27 35~38 73~8 27~92
[0122] 2. Preparation of the solution
[0123] Preparation of the reference substance solution of the index components: take about 5 mg of the reference substance o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com