Cholesteric liquid crystal handwriting board and preparation method thereof
A technology of cholesteric liquid crystal and handwriting tablet, which is applied in optics, instruments, electrical digital data processing, etc., and can solve problems such as poor adhesion, natural phase separation that does not achieve the best effect, and unsatisfactory brightness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] In the present invention, the preparation method of the liquid crystal layer feed solution preferably includes the following steps:
[0048] The polymerized monomer, photoinitiator, chiral agent, spacer and liquid crystal are mixed, then heated to above the clearing point, and stirred for 2 hours in the dark to obtain the liquid crystal layer feed solution.
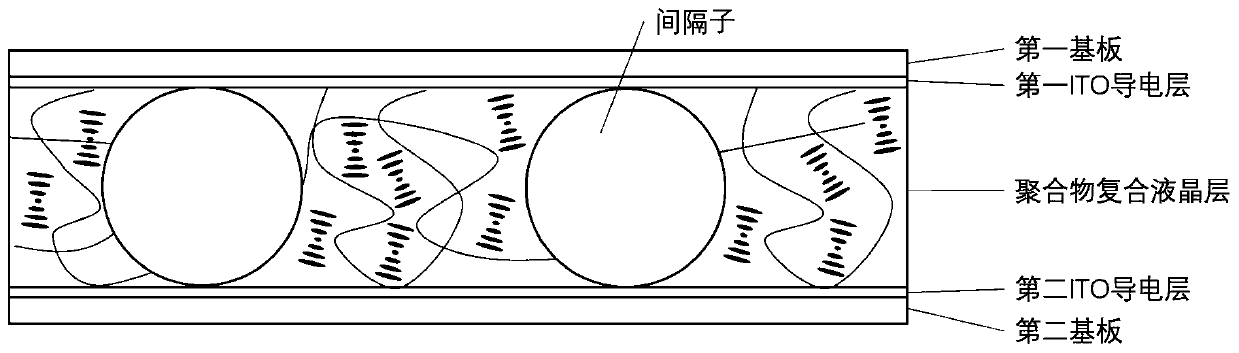
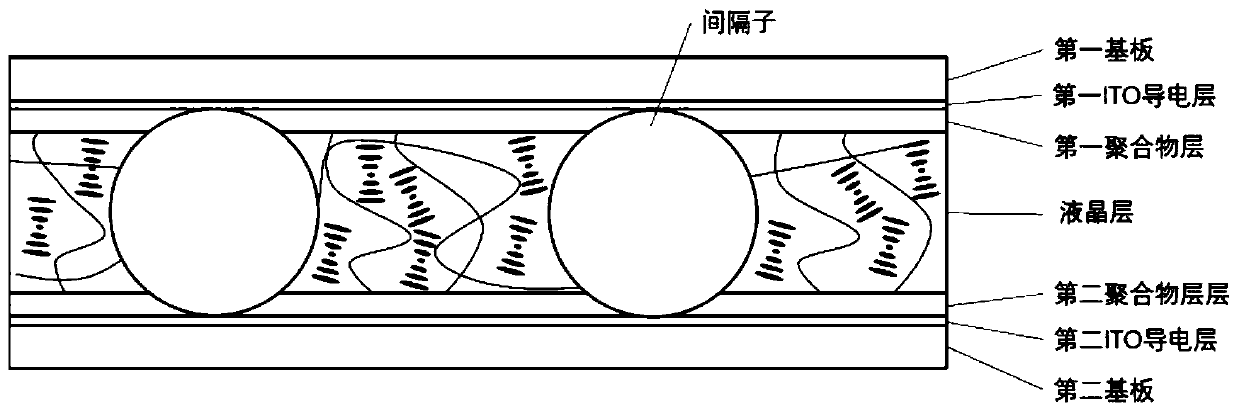
[0049] image 3 Schematic diagram of the structure of the cholesteric liquid crystal tablet provided by the present invention.
[0050] The present invention also provides the preparation method of the cholesteric liquid crystal tablet described in the above technical scheme, comprising the following steps:
[0051] Adhering the first ITO conductive layer and the first polymer layer in sequence on the first substrate, and attaching the second ITO conductive layer and the second polymer layer in sequence on the second substrate to obtain two splints;
[0052] The liquid crystal layer material is dropped on one end...
Embodiment 1
[0062] A preparation method for a cholesteric liquid crystal tablet, comprising the following steps:
[0063] The first ITO conductive layer (thickness is 100nm) is attached on the first substrate (material is PET) with a thickness of 50 μm, then the polyamic acid aqueous solution is dropped on the first ITO conductive layer, spin coating (rotating speed 3500r / min, time 30s) after heating at 220° C. for 10 minutes, attach a first polymer layer (polyimide) with a thickness of 1 μm on the first ITO conductive layer to obtain a splint, and obtain another splint according to the same method;
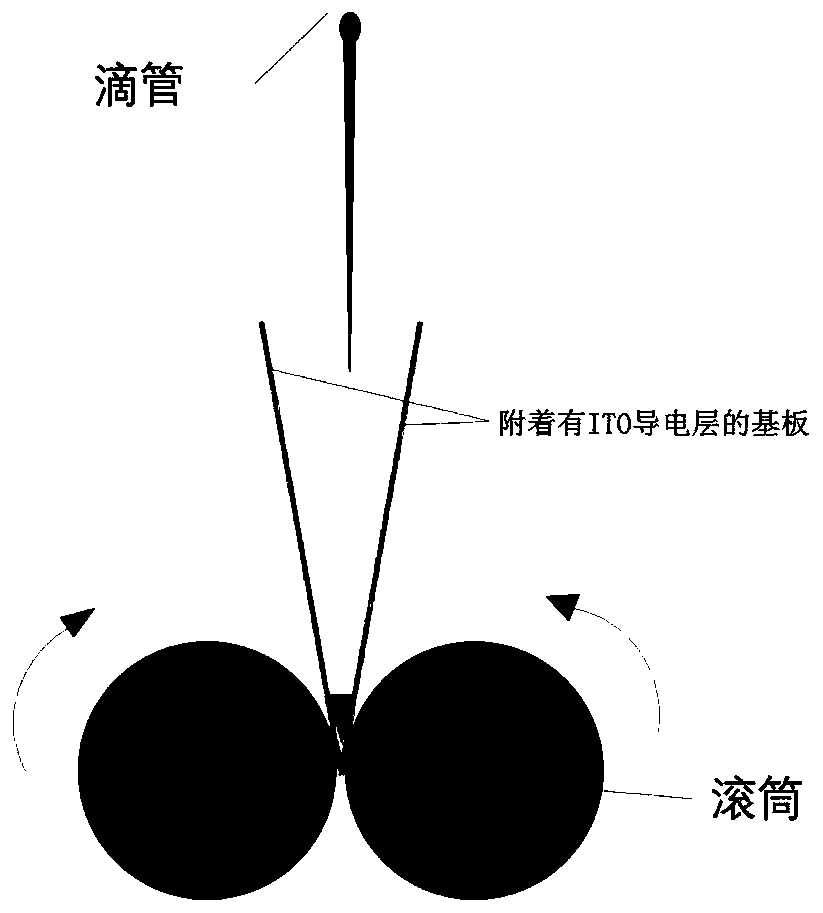
[0064] 5 parts by weight of RM257 monomer, 1.2 parts by weight of photoinitiator BME, 0.8 parts by weight of chiral agent R6N, 2 parts by weight of PS (with a particle diameter of 5 μm) and 100 parts by weight of liquid crystal E7 were mixed, then heated to above the clearing point, and Stir in the dark for 2 hours, drop the obtained liquid crystal layer material on one end of the two splint...
Embodiment 2
[0066] A preparation method for a cholesteric liquid crystal tablet, comprising the following steps:
[0067] A first ITO conductive layer (thickness 100 nm) was attached on a first substrate (material: PET) with a thickness of 50 μm.
[0068] 10 parts by weight of RM257 monomer, 1.2 parts by weight of photoinitiator BME, 0.8 parts by weight of chiral agent R6N, 2 parts by weight of PS (with a particle diameter of 5 μm) and 100 parts by weight of liquid crystal E7 were mixed, then heated to above the clearing point, and Stir in the dark for 2 hours, drop the obtained liquid crystal layer on one end of the two splints, squeeze it from one end of the splint to the other end with a roller, repeat extrusion 3 times, heat to 100°C and then ultraviolet light (intensity 2mW / cm 2 ) to polymerize for 20min to obtain a polymer layer (an olefin polymer whose side group is an ester group), then stop the light, cool naturally to room temperature, and then use ultraviolet light (intensity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com