Method for determining impurities in candesartan cilexetil
A technology for candesartan medoxomil and impurities, which is applied in the field of food and drug safety testing, can solve the problems of insufficient pertinence of impurity control, poor accuracy, and difficulty in popularizing detection methods, and achieves long purchasing cycle, strong pertinence and good quality. The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
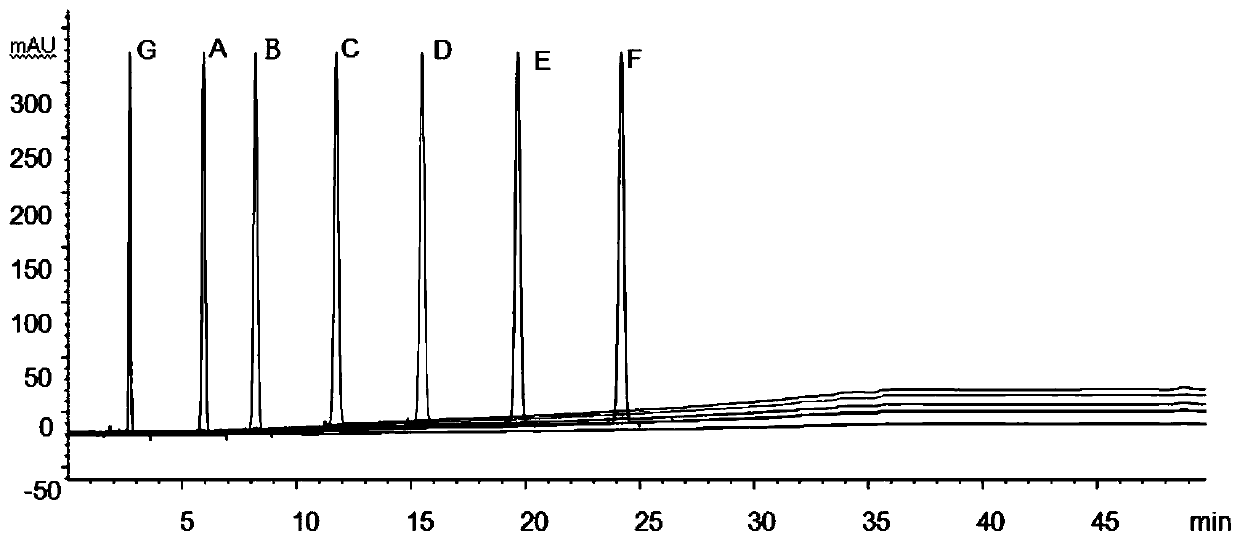
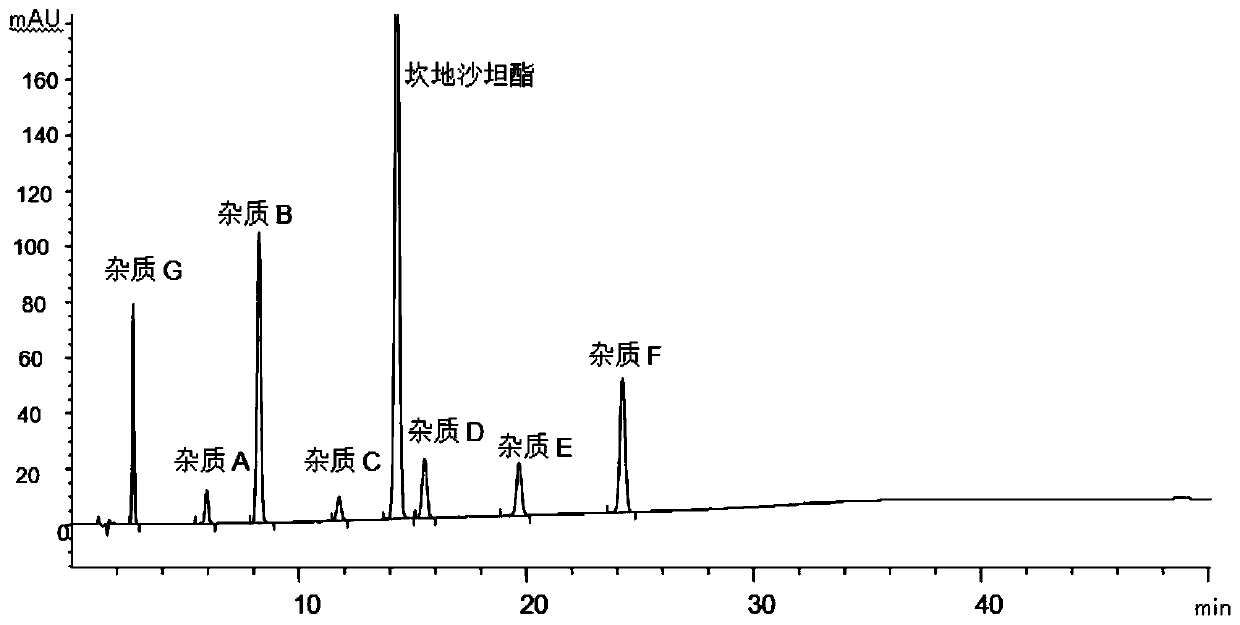
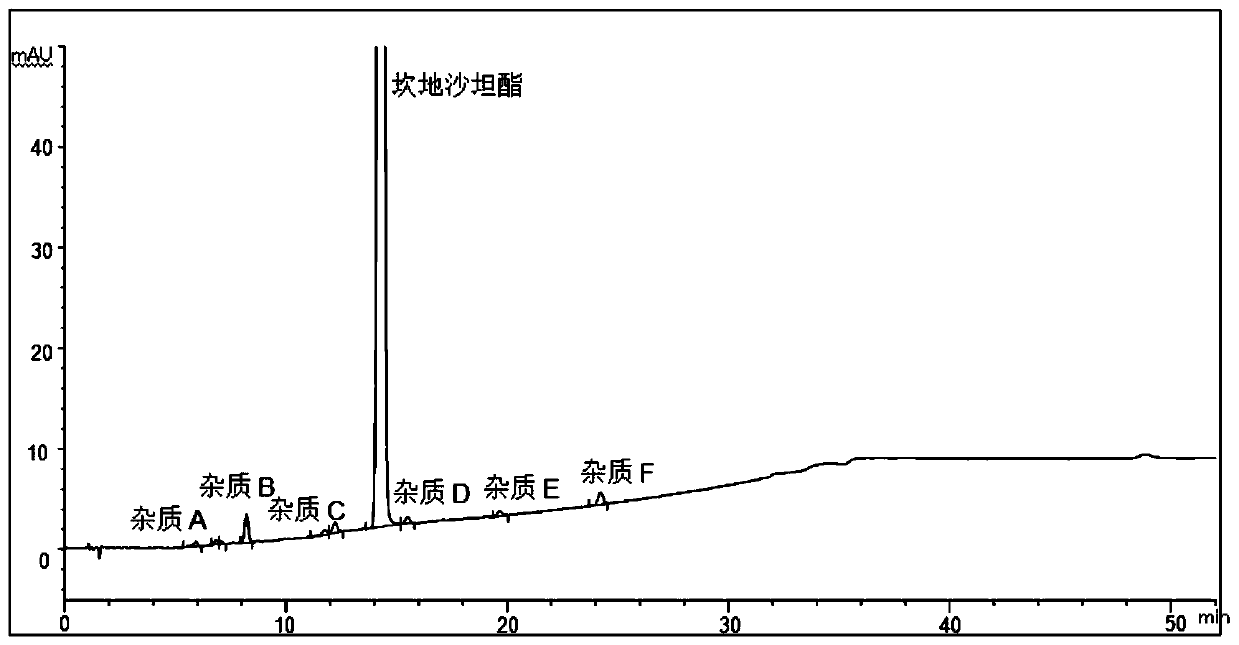
[0051]The determination of impurity in embodiment 1 candesartan cilexetil
[0052] The structural formula of impurities (impurity A, B, C, D, E, F or G) in candesartan cilexetil and candesartan cilexetil is shown in Table 1, wherein, impurity A is a synthetic by-product, impurity B, C, D, E, and F are impurities, and impurity G is a synthetic starting material, which will be degraded to varying degrees under various damage conditions; according to the structural characteristics of the product, impurities B, C, D, E, and F are candi The main degradation impurity of sartan cilexetil.
[0053] Table 1 candesartan cilexetil and the structural formula of impurities in candesartan cilexetil
[0054]
[0055]
[0056] Through the destruction experiment, adopting candesartan cilexetil standard substance can destroy impurity A, B, C, D, E, F, G, through repeated experimentation, adopt the measurement of impurity in the candesartan cilexetil provided by this embodiment 1 Methods...
Embodiment 2
[0080] The determination of impurity in embodiment 2 candesartan cilexetil
[0081] 1. Experimental method
[0082] A method for measuring impurities in candesartan cilexetil, comprising the following steps:
[0083] S1. Take 20mg of candesartan cilexetil standard product, put it in a graduated test tube, add 50mL of acetonitrile-water (volume ratio 3:2) mixed solution to dissolve, shake well, heat in a water bath (100°C) for 1.5h, take it out, and let it cool until At room temperature, add acetonitrile (50% by volume) to 50 mL, shake well to obtain degradation solution 1;
[0084] In addition, take 20 mg of candesartan cilexetil standard product, put it in a 50 mL volumetric flask, dissolve it with ethanol, add 25 mL of 0.5 mol / L calcium hydroxide solution, let it stand for 15 min, add 2.5 mL of 0.5 mol / L sulfuric acid for neutralization, and then dissolve it with ethanol Constant volume to obtain degradation solution 2; take degradation solution 1 and degradation solution ...
Embodiment 3
[0093] The determination of impurity in embodiment 3 candesartan cilexetil
[0094] 1. Experimental method
[0095] A method for measuring impurities in candesartan cilexetil, comprising the following steps:
[0096] S1. Take 40 mg of candesartan cilexetil standard (purchased from China National Institutes for Food and Drug Control), put it in a graduated test tube, add 100 mL of acetonitrile-water (volume ratio 3:2) mixed solution to dissolve, shake well, and heat in a water bath (100 ℃) for 3 hours, take it out, let it cool to room temperature, add acetonitrile (50% by volume) to 100mL, shake well, and obtain degradation solution 1;
[0097]In addition, take 10 mg of candesartan cilexetil standard product, put it in a 25 mL volumetric flask, dissolve it with ethanol, add 25 mL of 0.5 mol / L potassium hydroxide solution, let it stand for 15 min, add 2.5 mL of 0.5 mol / L nitric acid for neutralization, and then dissolve it with ethanol Constant volume to obtain degradation sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com