Preparation method of P2Y2 receptor stimulant diquafosol tetrasodium
The technology of a receptor agonist and tetrasodium fossodium is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc. It can solve the problems of high price, low economic benefit, and scale-up, and achieve easy industrial production, Simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
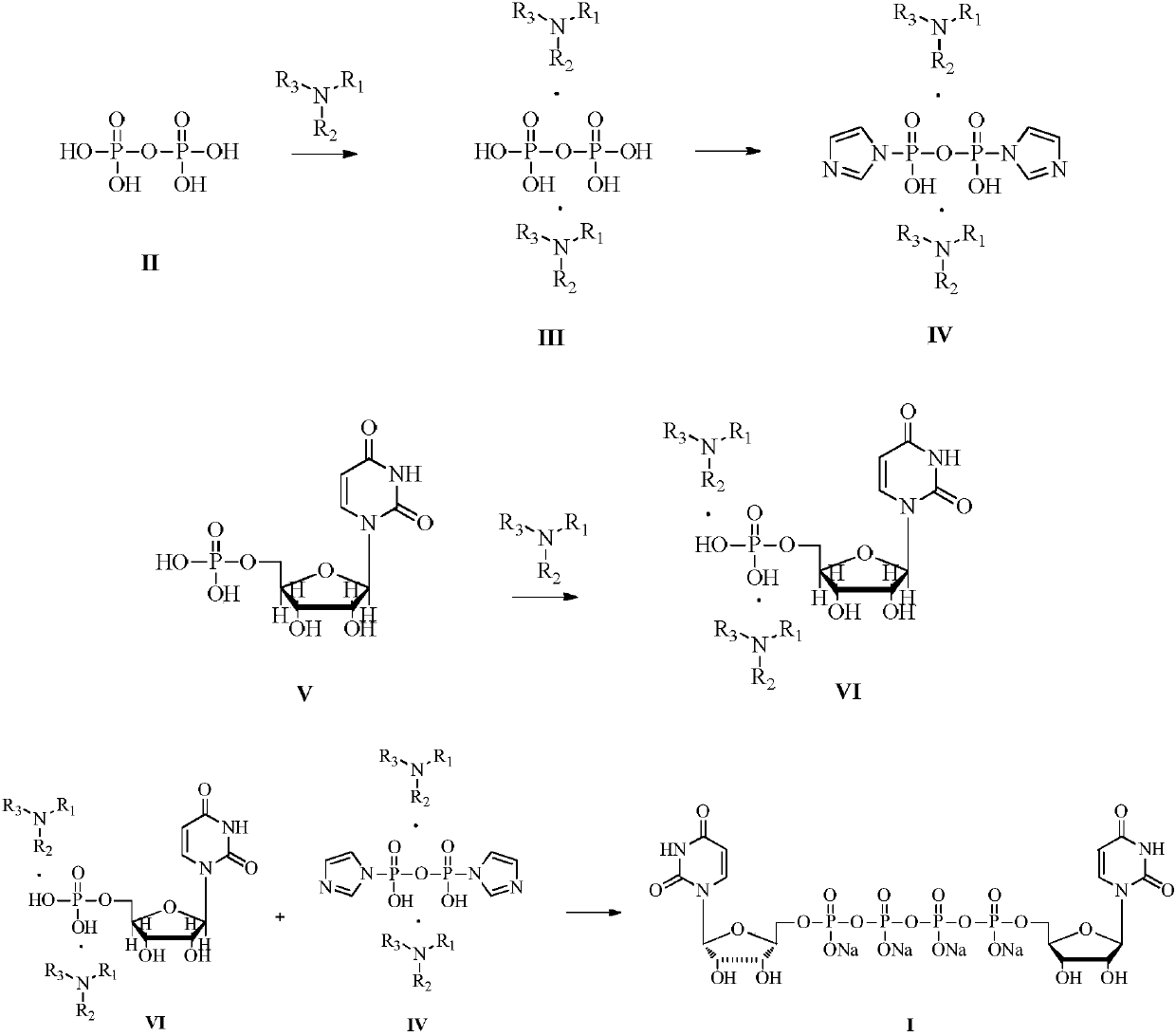
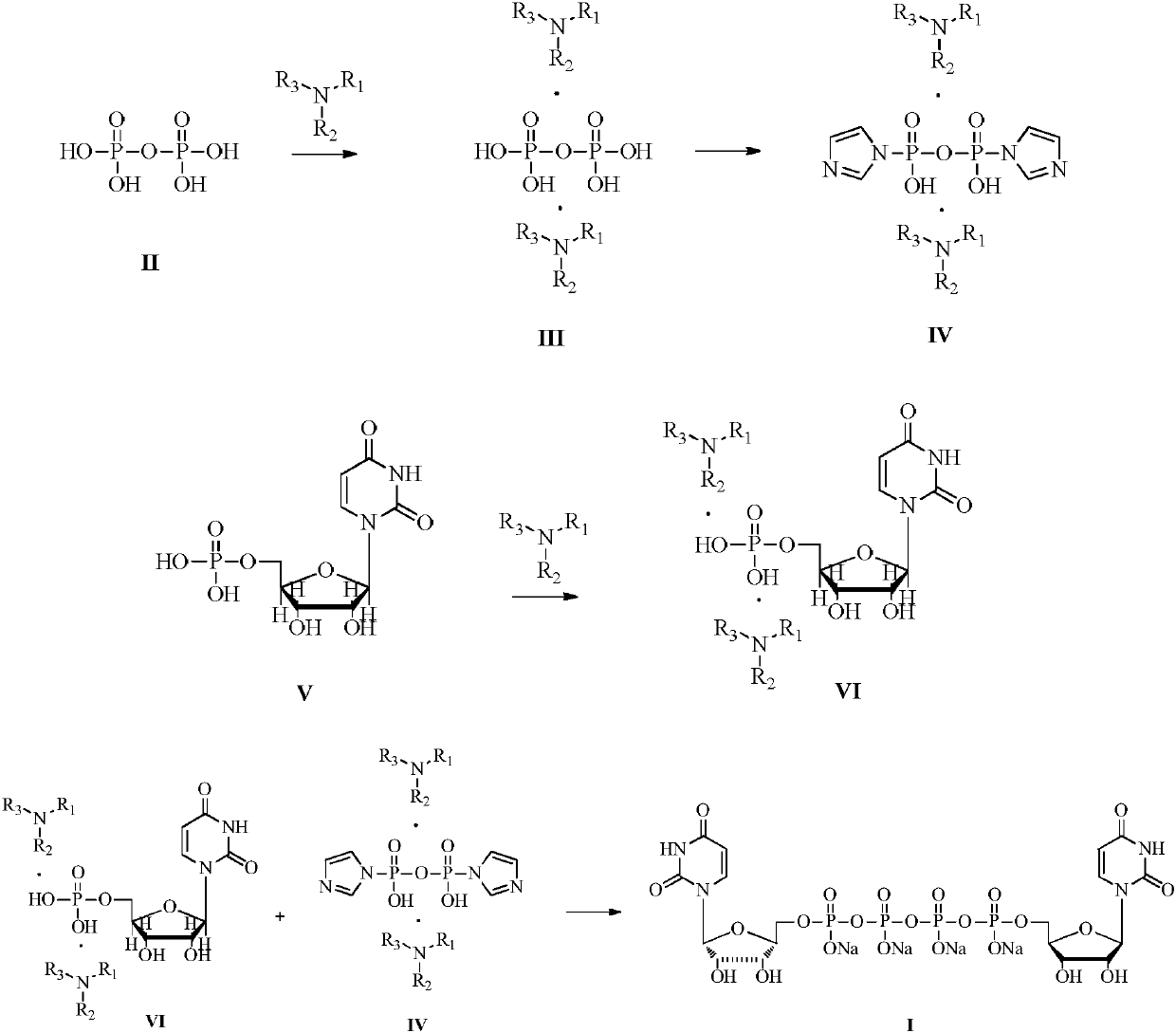
[0018] Embodiment 1: Preparation of uridine monophosphate diisobutylbutylamine salt
[0019]
[0020] Uridine monophosphate disodium salt (1000g, 2.72mol) was dissolved in deionized water (10L). Butylamine (1007.6g, 5.44mol) was salified, and after stirring at room temperature for 12 hours, the above-mentioned solution that had been concentrated into a salt in vacuo became an oil, and its water content was controlled below 1%. The oily substance of uridine monophosphate diisobutylbutylamine salt was obtained, and 3 L of DMF was added to dissolve the oily substance to obtain a DMF solution of uridine monophosphate diisobutylbutylamine salt, which was stored for future use.
Embodiment 2
[0021] Embodiment 2: Preparation of diisobutylbutylamine pyrophosphate
[0022]
[0023] Tetrasodium pyrophosphate (361.6g, 1.36mol) was dissolved in deionized water (10L), the aqueous solution was passed through a strong acid type cation exchange resin (Amberlite 732 proton type), the effluent containing pyrophosphate was combined, and diisobutyl Butylamine (503.8g, 2.72mol) was salted, and after stirring at room temperature for 12 hours, the above solution that had been concentrated into a salt in vacuo became an oil, and its water content was controlled below 1%. The oily substance of diisobutylbutylamine pyrophosphate was obtained, and 3 L of DMF was added to dissolve the oily substance to obtain a DMF solution of diisobutylbutylamine pyrophosphate, which was stored for future use.
Embodiment 3
[0024] Embodiment 3: Preparation of imidazole diisobutylbutylamine pyrophosphate
[0025]
[0026] In the DMF solution of diisobutylbutylamine pyrophosphate, add 1,1-carbonyldiimidazole (441.1 g, 2.72 mol) as an activator, stir at room temperature for 6 hours, add excess methanol to quench the unreacted CDI, Stir at room temperature for 1 hour, then concentrate in vacuo to remove excess methanol, and obtain a DMF solution of imidazole diisobutylbutylamine pyrophosphate and store it for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com