Carrageenin in-situ forming gel spray for noses, and preparation method of carrageenin in-situ forming gel spray for noses
A technology of spray and carrageenan, which is applied in the field of medicine, can solve the problems of inconvenient use of nasal administration and irritation of nasal mucosa, etc., and achieve the effect of enhancing the ability to prevent respiratory infectious diseases, enhancing stimulation, and preventing syneresis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
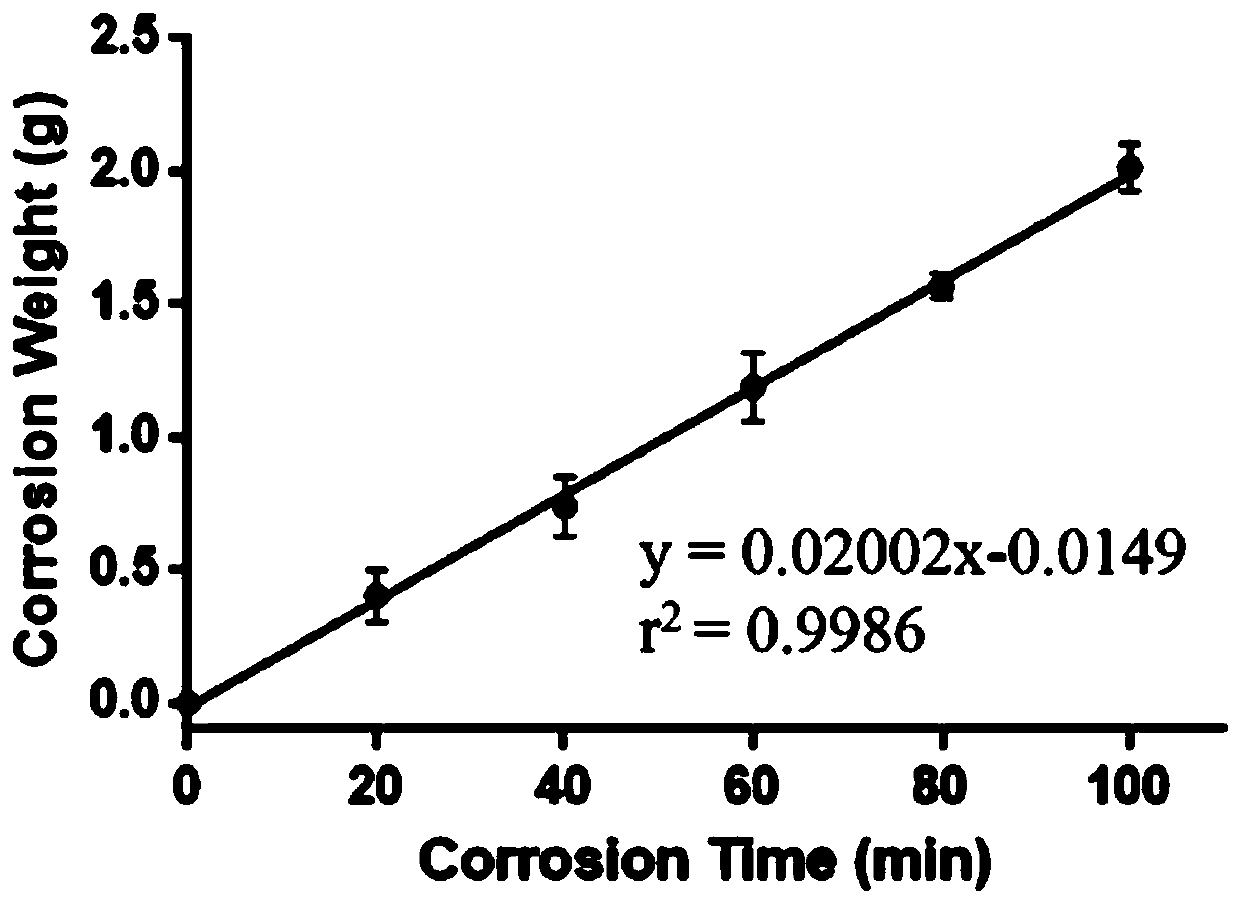
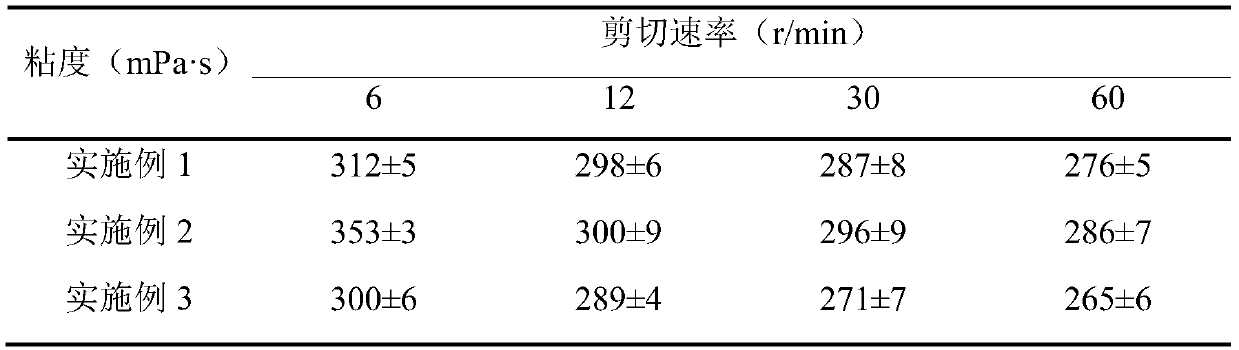
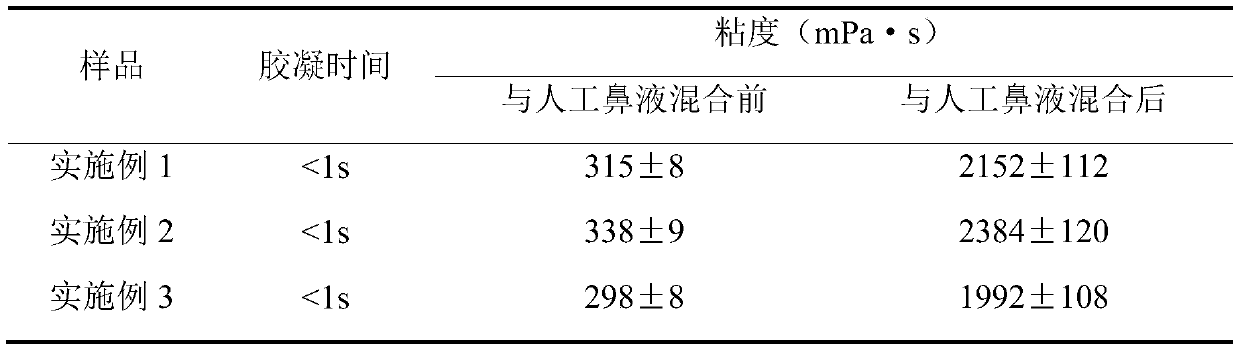
Image
Examples
Embodiment 1
[0024] A carrageenan nasal instant gel spray, by weight percentage, the gel spray comprises the following components: 6% of κ-type carrageenan with a molecular weight of 30k, 4% of iota-type carrageenan with a molecular weight of 30k, 0.001% of λ-type carrageenan with a molecular weight of 20k, 1% of antiviral drug (interferon), acceptable excipients in external skin preparations (suspending agent [xanthan gum] 0.3%, wetting agent [glycerin] 10%, Preservative [chlorobutanol] 0.3%, pH adjuster [sodium hydroxide, hydrochloric acid]) 10.6%, and the balance is deionized water. This gel spray is prepared as follows:
[0025] The κ-type carrageenan with a molecular weight of 30k, the iota-type carrageenan with a molecular weight of 30k, the lambda-type carrageenan with a molecular weight of 20k, antiviral drugs (interferon), and acceptable adjuvant (suspending agent [yellow] Raw gum], wetting agent [glycerin], preservative [chlorobutanol]) are added to deionized water, mixed well a...
Embodiment 2
[0027] A carrageenan nasal instant gel spray, by weight percentage, the gel spray comprises the following components: 7% of κ-type carrageenan with a molecular weight of 20k, 3% of iota-type carrageenan with a molecular weight of 40k, Molecular weight is 0.005% of lambda type carrageenan of 10k, antiviral drug (amantadine hydrochloride) 1%, acceptable adjuvant (suspending agent [carboxymethylcellulose sodium] 5%, wetting agent [ Tween 60] 8%, preservative [ethylparaben] 0.3%, pH regulator [ethanolamine, citric acid]) 13.3%, and the balance is deionized water. This gel spray is prepared as follows:
[0028] The κ-type carrageenan with a molecular weight of 20k, the iota-type carrageenan with a molecular weight of 40k, the λ-type carrageenan with a molecular weight of 10k, antiviral drugs (amantadine hydrochloride), acceptable adjuvant (suspension agent) in external skin preparations [Sodium carboxymethylcellulose], wetting agent [Tween 60], preservative [ethylparaben]) are add...
Embodiment 3
[0030] A carrageenan nose instant gel spray, by weight percentage, the gel spray comprises the following components: 5% of κ-type carrageenan with a molecular weight of 40k, 5% of iota-type carrageenan with a molecular weight of 20k, 0.001% of λ-type carrageenan with a molecular weight of 10k, 1.5% of antiviral drug (Ateclovir), acceptable adjuvant in skin topical preparations (suspending agent [Carbopol] 5%, wetting agent [ethanol] 5% %, preservative [potassium sorbate] 1%, pH regulator [ethylenediamine, sorbic acid]) 11%, and the balance is deionized water. This gel spray is prepared as follows:
[0031] The κ-type carrageenan with a molecular weight of 40k, the iota-type carrageenan with a molecular weight of 20k, the λ-type carrageenan with a molecular weight of 10k, antiviral drugs (ateclovir), acceptable adjuvant (suspension agent) in skin external preparations [Carbopol], wetting agent [ethanol], preservative [potassium sorbate]) into deionized water, mix well and add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com