Application of rhodanine to treatment of metabolic diseases
A technology of derivatives and drugs, applied in the field of application of rhodanine derivatives in the treatment of metabolic diseases, can solve problems such as side effects and ineffective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, in vitro verification
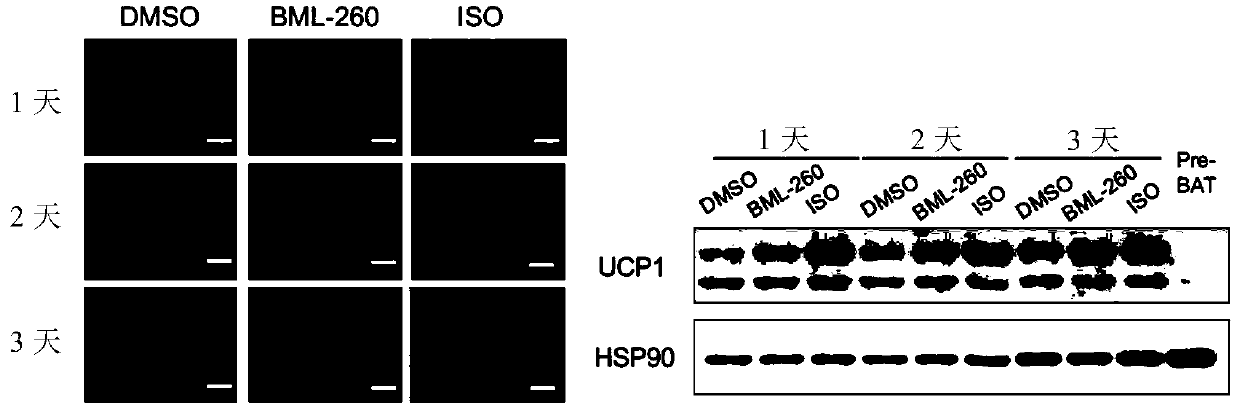
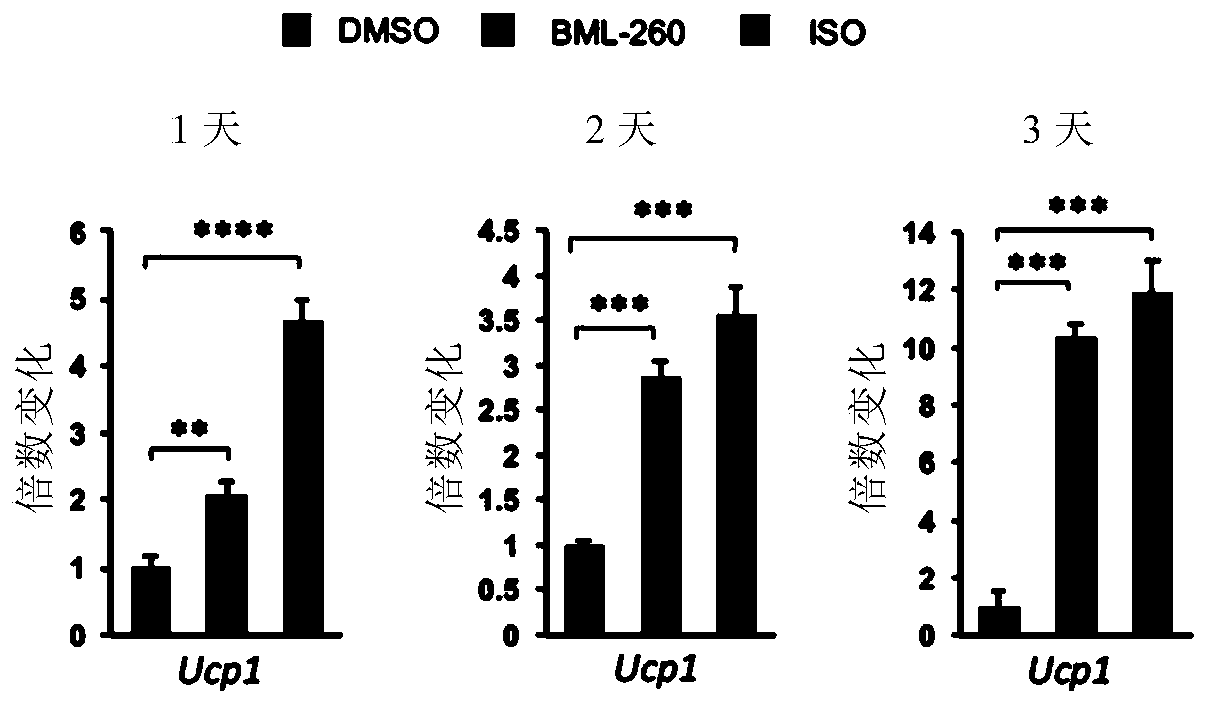
[0075] 1) BML-260 can promote the expression of UCP1 in mature brown adipocytes
[0076]Experimental procedure: Ucp1-2A-GFP primary brown adipocytes that have been successfully constructed are used (the 2A-GFP sequence is inserted after the Ucp1 stop codon in situ in the cell, so that the expression of GFP can reflect the expression level of endogenous UCP1 protein to a certain extent , the inserted 2A-GFP sequence is:, SEQ ID NO: 1), adding BML-260 at different stages of differentiation for different time. Three compound treatment methods were designed: adding BML-260 (10 μM) on day 1 for 10 days (re-adding 10 μM BML-260 when changing the medium); on day 7 of differentiation (mature brown adipocyte stage ) were treated with BML-260 (10 μM) for 1 day, 2 days, and 3 days respectively; BML-260 (10 μM) was only treated on the 1st to 3rd day of differentiation (differentiation induction stage), and samples were collected on the 10th da...
Embodiment 2
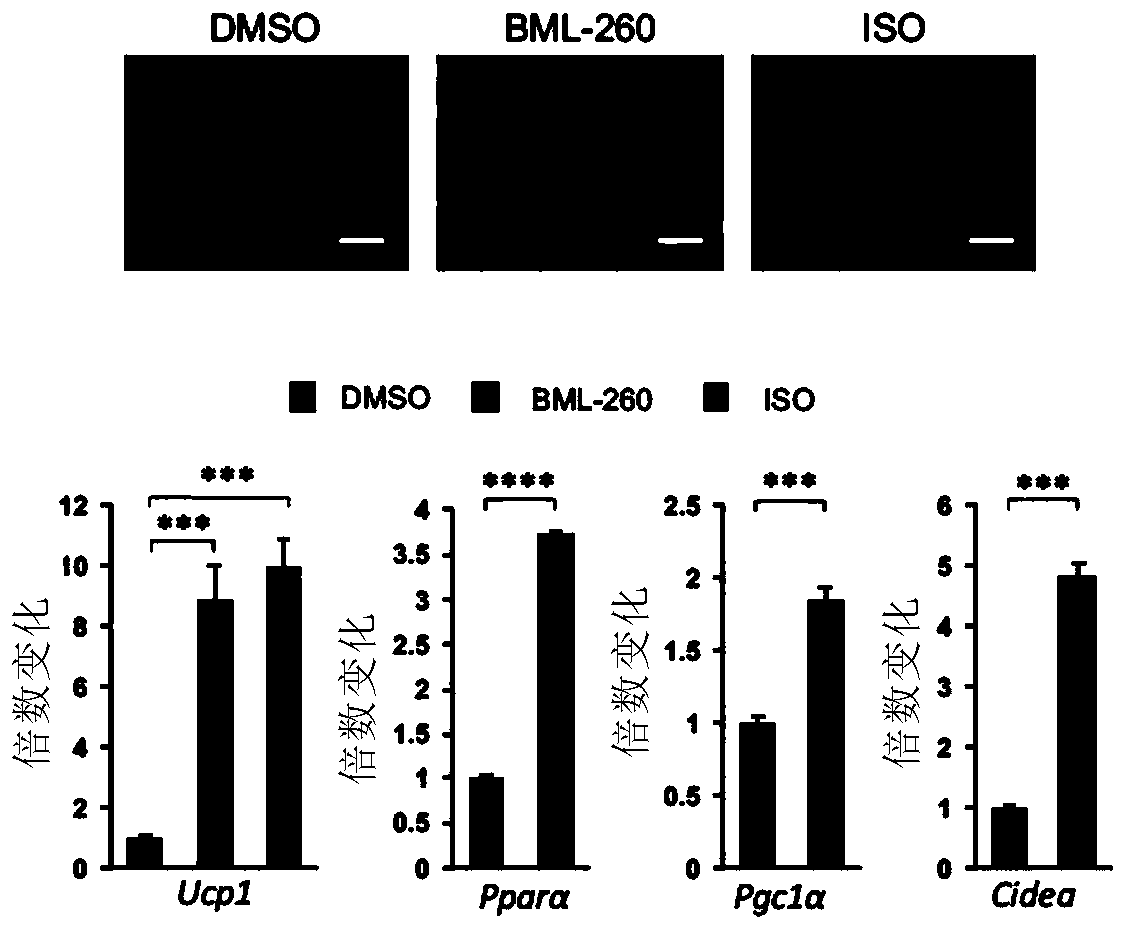
[0096] Embodiment 2, verification in vivo
[0097] 1) BML-260 activates the expression of UCP1 in subcutaneous white fat of mice and increases thermogenesis
[0098] Experimental procedure: eight-week-old C57BL / 6J male mice (Slack) were orthotopically injected with BML-260 in the subcutaneous fat, the solvent was used as a negative control, and CL-316243 (CL) was used as a positive control. Three days after the injection, the mice were weighed, the subcutaneous fat of the mice was taken and weighed, and the protein was extracted for Western blot to detect the expression of UCP1. And the mouse subcutaneous fat was embedded in paraffin, and hematoxylin-eosin staining (HE-staining) or UCP1 protein immunohistochemistry was performed to observe the morphology of adipose tissue and the expression of UCP1 in situ. After the in situ injection, a cold stimulation experiment was performed to test the tolerance of the mice to continuous low temperature.
[0099] Experimental results su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com