Method for electric field enhanced selective adsorption of lead ions in heavy metal wastewater
A technology for adsorbing heavy metals and selectivity, which is applied in separation methods, chemical instruments and methods, and water pollutants. It can solve the problems of poor selectivity of adsorbents, difficulty in recovering heavy metals, and low recovery purity, achieving enhanced adsorption selectivity, Enhanced selectivity and reduced contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0016] Specific embodiment 1: This embodiment is a method for electric field enhanced selective adsorption of lead ions in heavy metal wastewater, specifically carried out according to the following steps:
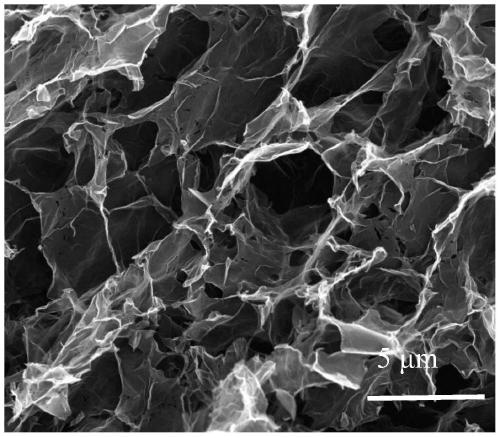
[0017] 1. Tannic acid@graphene oxide conductive airgel is used as the working electrode, Ag / AgCl is used as the reference electrode, and platinum mesh is used as the counter electrode to form a three-electrode system. The sodium nitrate aqueous solution is used as the electrolyte solution, and the current-time method is used. Carry out electrical reduction, the applied voltage is -1.2V~-2V, and the reduction time is 2min~30min to obtain tannic acid@reduced graphene oxide conductive airgel; the concentration of the sodium nitrate aqueous solution is 0.5mol / L~0.6 mol / L;
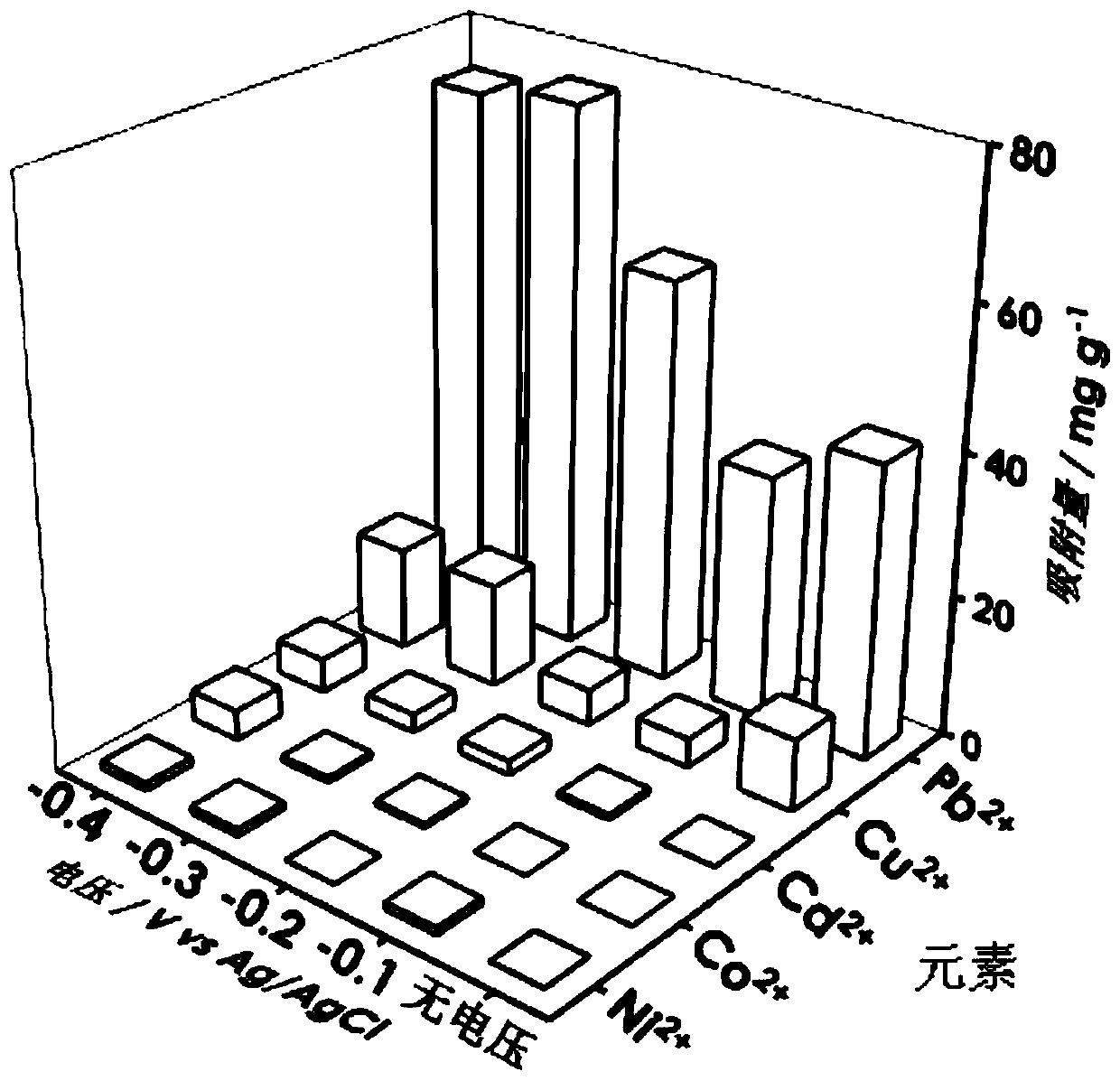
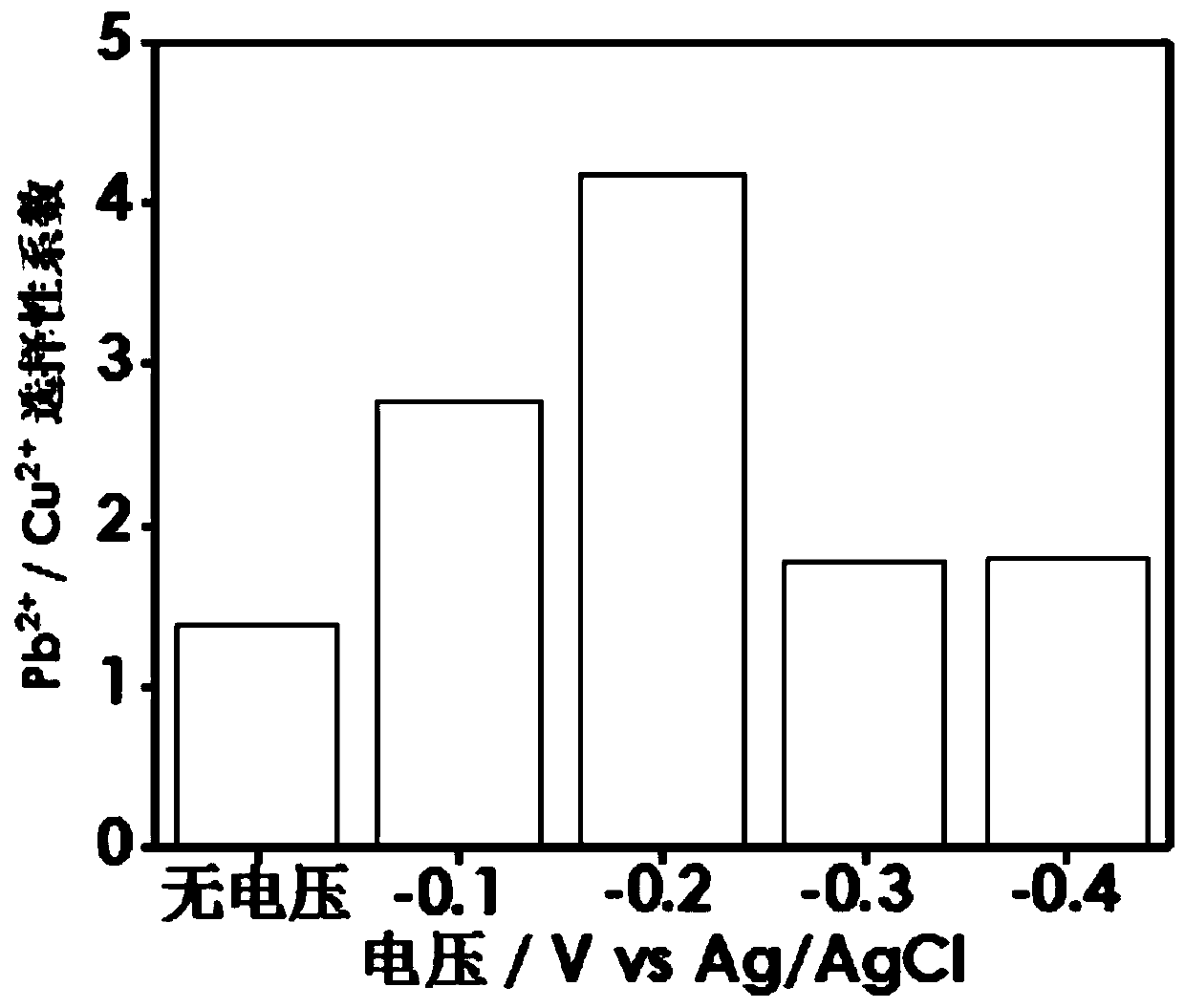
[0018] 2. The tannic acid@reduced graphene oxide conductive airgel prepared in step 1 was used as the working electrode, Ag / AgCl was used as the reference electrode, and the platinum mesh was used as the co...
specific Embodiment approach 2
[0019] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the preparation method of the tannic acid@graphene oxide conductive airgel described in step one is as follows:
[0020] Mix 2.5mL of graphene oxide dispersion liquid and 1mL of tannic acid aqueous solution evenly, ultrasonically disperse for 20min, then add 1.5mL of deionized water, ultrasonically disperse for 10min, put it in an oven at 90°C for 20h, take it out from the oven Soak in deionized water for 3 minutes, change to clean deionized water every 30 minutes, and continue to soak for 30 minutes until the aqueous solution changes from light yellow to colorless and transparent to wash away excess tannic acid; finally freeze-dry for 24 hours, That is, tannic acid@graphene oxide conductive airgel is obtained;
[0021] The concentration of the graphene oxide dispersion is 4 mg / mL, and the solvent is deionized water, purchased from Bailingwei Technology Co., Ltd.;
[0022] T...
specific Embodiment approach 3
[0023] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that in step 1, an electrochemical workstation CHI760E is used for electroreduction using the current-time method. Others are the same as those in Embodiment 1 or 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com