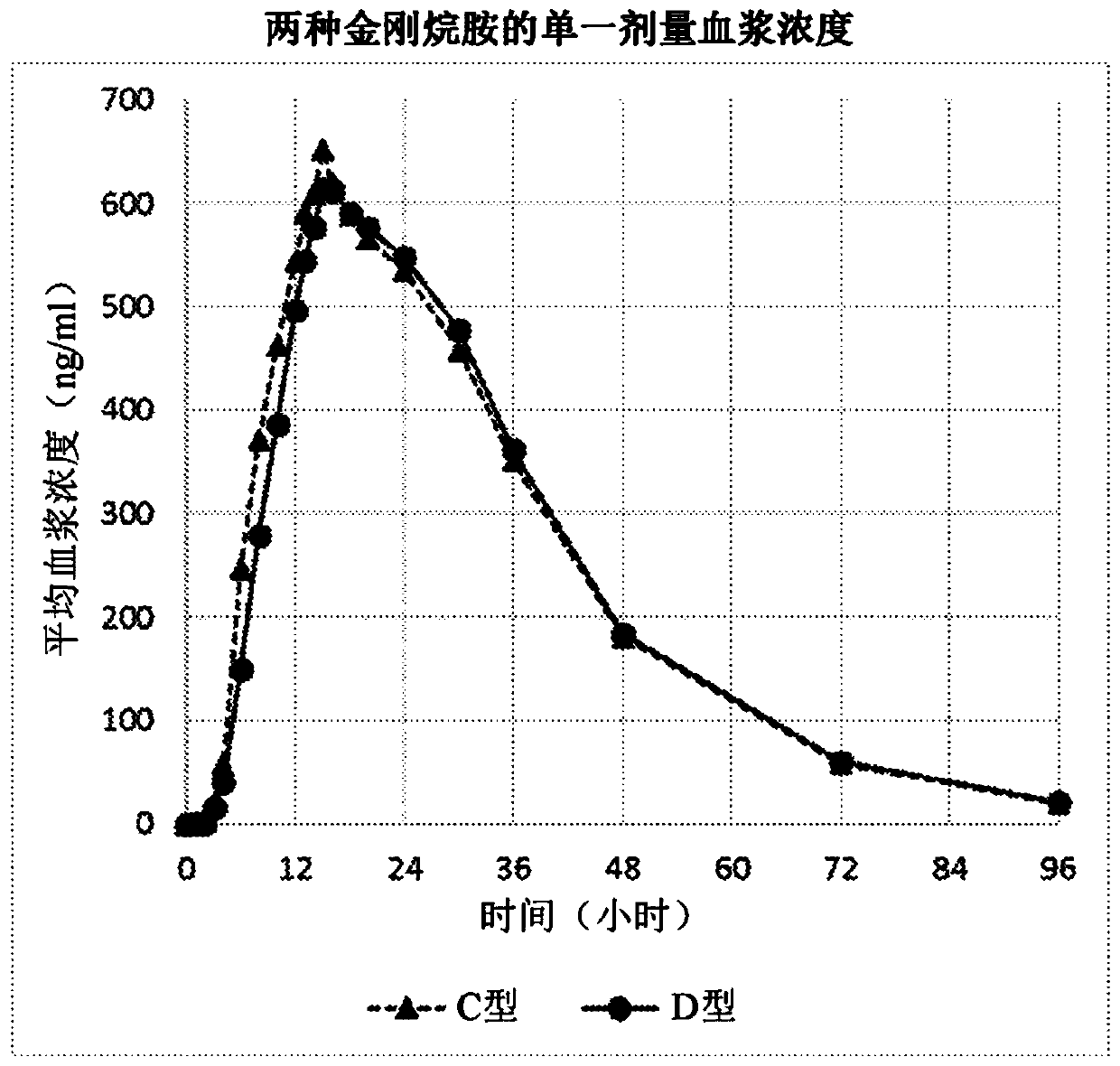
Amantadine compositions, preparations thereof, and methods of use
A kind of technology of amantadine and composition, applied in the field of sustained-release oral pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-1
[0224] Embodiment I-1. A kind of oral pharmaceutical composition, it comprises:
[0225] 137 mg to 500 mg of amantadine or a pharmaceutically acceptable salt thereof, and
[0226] at least one excipient which modifies the release of the drug,
[0227] wherein the pharmaceutical composition has a Tmax of amantadine of (i) 11 to 19 hours when administered to healthy individuals in a single dose, fasting human pharmacokinetic study, (ii) 44 AUC of amantadine to 72ng*hr / ml / mg of the drug 0-inf , and (iii) the pAUC of amantadine from 1.0 to 2.0 ng*hr / ml / mg of the drug 0-8 .
Embodiment I-2
[0228] Embodiment 1-2. A kind of oral pharmaceutical composition, it comprises:
[0229] 137 mg to 500 mg of amantadine or a pharmaceutically acceptable salt thereof, and
[0230] at least one excipient that modifies the release of said amantadine or said pharmaceutically acceptable salt thereof;
[0231] wherein the pharmaceutical composition has a Tmax of amantadine of (i) 11 to 19 hours when administered to healthy individuals in a single dose, fasting human pharmacokinetic study, (ii) 44 AUC of amantadine to 72ng*hr / ml / mg of the drug 0-inf , and (iii) the pAUC of amantadine from 0.3 to 0.9 ng*hr / ml / mg of the drug 0-6 .
Embodiment I-3
[0232] Embodiment 1-3. The oral pharmaceutical composition according to embodiment 1-1, wherein when administered to healthy individuals in a single dose, fasting human pharmacokinetic study, the oral pharmaceutical composition has a concentration of 0.3 to pAUC of amantadine at 0.9ng*hr / ml / mg of the drug 0-6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com