Preparation method of 4-amino-1, 3-dihydro-benzimidazole-2-one
A technology of benzimidazole and dihydrogen, applied in the field of organic compound synthesis and pharmaceuticals, can solve the problems of large side effects, low final yield, slow reaction process, etc., achieve good social and economic benefits, great economic value potential, and easy reaction process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
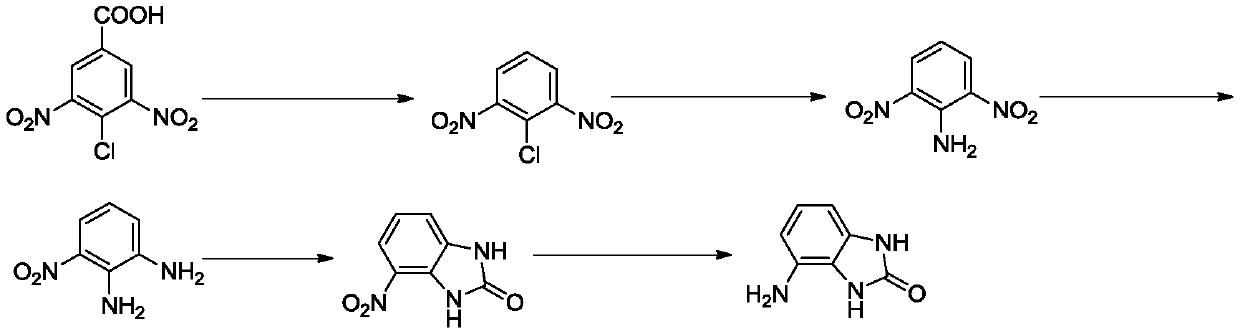
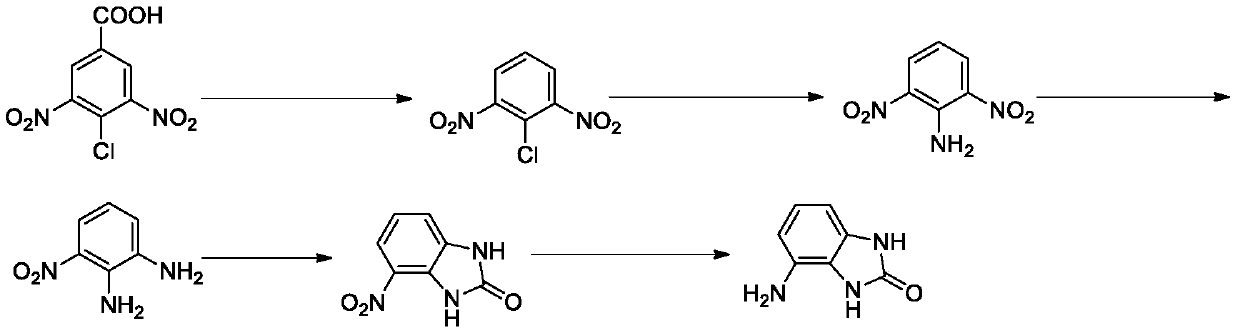
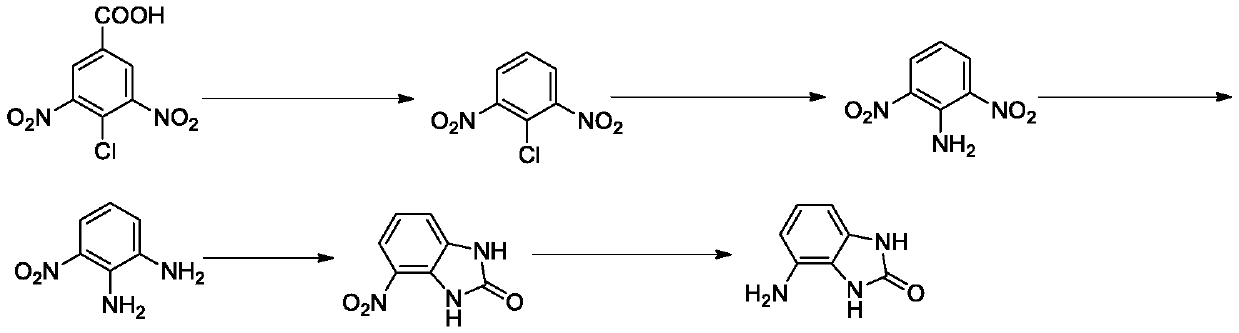
[0031] Its preparation method comprises the following steps:
[0032] Q1, the preparation of 2,6-dinitrochlorobenzene, under the reaction temperature, 3,5-dinitro-4-chlorobenzoic acid is used as raw material, and decarboxylated in the reaction solvent to form 2,6-dinitrochlorobenzene , the reaction temperature is 180-200°C, and the reaction time is 1-3 hours;
[0033] Q2, the preparation of 2,6-dinitroaniline, react 2,6-dinitrochlorobenzene with ammonia water, the reaction temperature is 90-110°C, the reaction time is 2-5 hours, then extraction, drying, column layer Analysis and separation obtain 2,6-dinitroaniline;
[0034] Q3. The preparation of 3-nitro-o-phenylenediamine, the reduction reaction of 2,6-dinitroaniline, the reaction temperature is 50-80°C, and the reaction time is 1-3 hours to obtain 3-nitro-o-phenylenediamine ;
[0035] Q4. Preparation of 4-nitro-1H-benzo[d]imidazol-2(3H)-one, 3-nitro-o-phenylenediamine and triphosgene to generate 4-nitro-1H-benzo[d] Imid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com