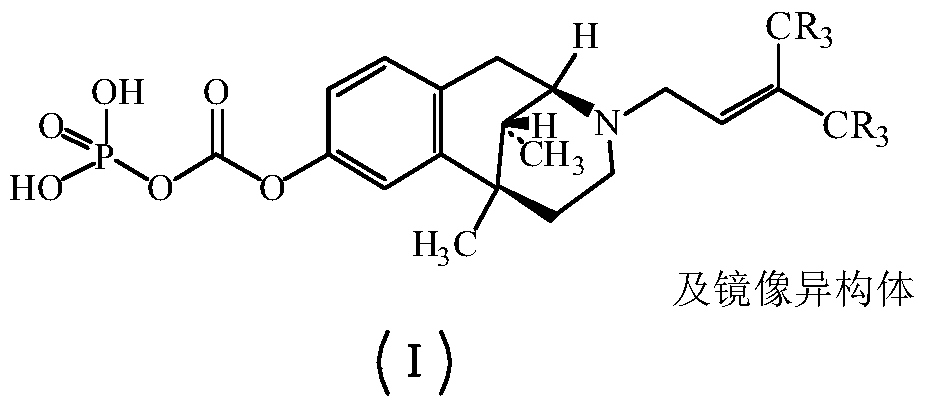
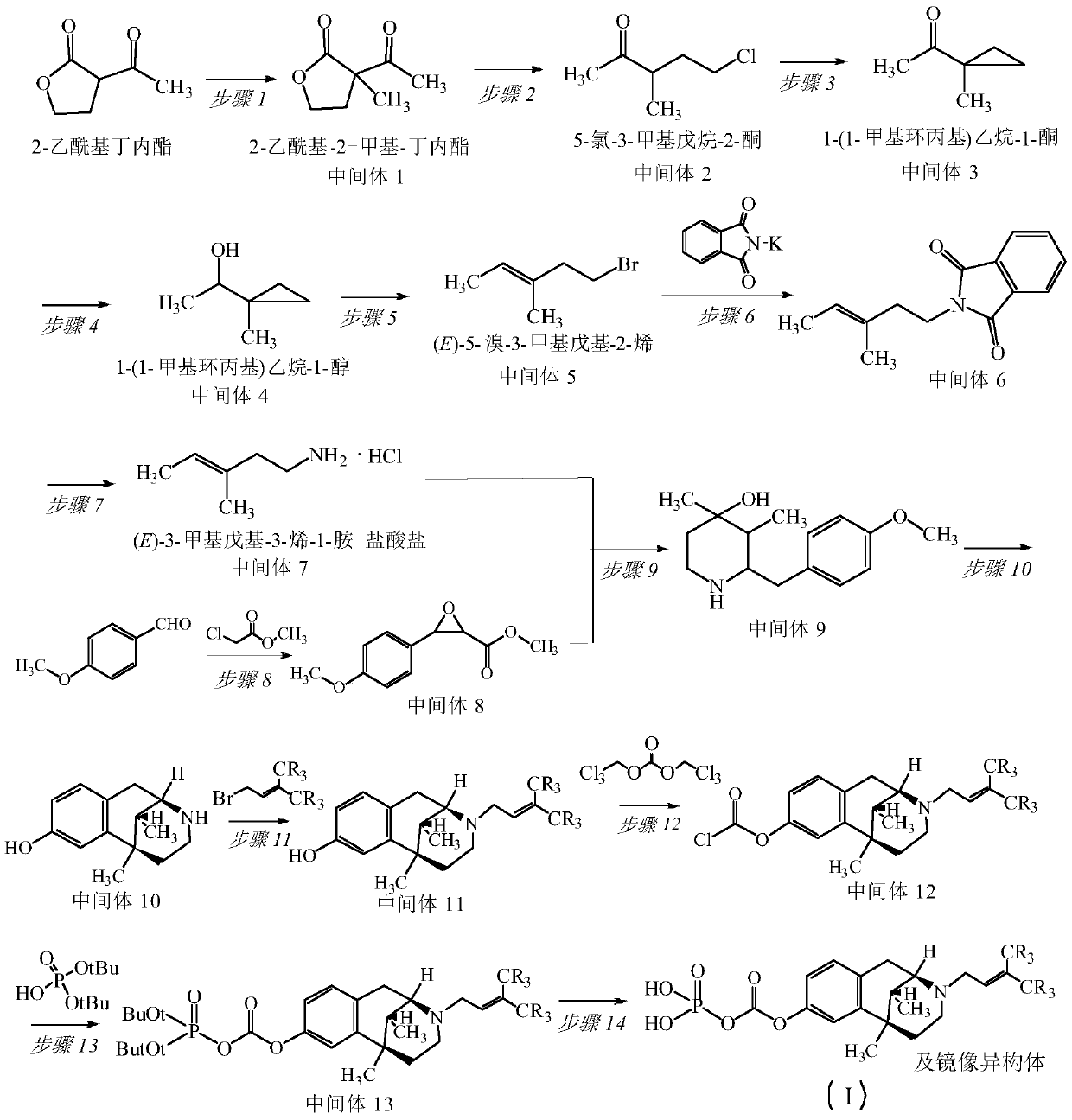
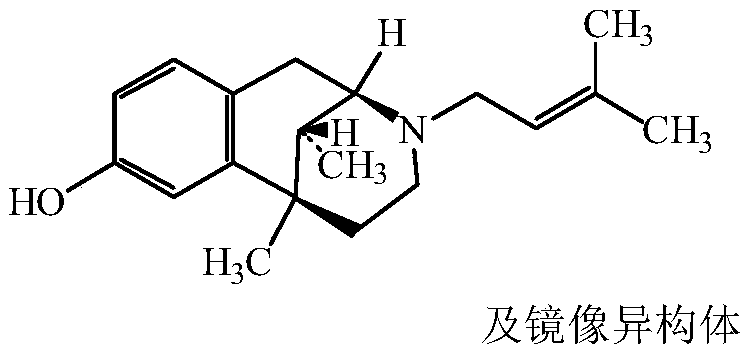
Pentazocine prodrug as well as preparation method and application thereof
A technology of pentazocine and prodrug, applied in the field of medicinal chemistry, can solve problems such as unsatisfactory, and achieve the effects of improving bioavailability, stable biological properties, and delaying drug action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthesis of embodiment 1 pentazocine prodrug (compound 1)
[0045]
[0046] 1, Synthesis of 2-acetyl-2-methyl-butyrolactone (intermediate 1)
[0047] N 2 For protection, a solution of 2-acetylbutyrolactone (51g, 0.4mol) in 400ml of anhydrous dichloromethane was added dropwise to [Me 2 Cl][Al(OTeF 5 ) 4 ] (0.8mol) solution, stirred at room temperature overnight, filtered, the solid was washed several times with an appropriate amount of dichloromethane, and concentrated under reduced pressure to obtain 51.7 g of light yellow oil, with a yield of 91.0%.
[0048] 2. Synthesis of 5-chloro-3-methylpentan-2-one (intermediate 2)
[0049] 2-acetyl-2-methyl-butyrolactone (51g, 0.359mol), concentrated hydrochloric acid (128ml, 32%, 1.07mol), 150ml of distilled water, heated carefully until the gas ceased, and the reaction compound was distilled to collect 140ml of distillate, The residue was added to 90ml of distilled water, and 80ml of distillate was collected by dis...
Embodiment 2
[0082] Synthesis of embodiment 2 deuterated pentazocine prodrug (compound 2)
[0083]
[0084] With embodiment 1, wherein the synthesis of intermediate 11, use [4,4'- 2 h 6 ]-1-bromo-3-methyl-2-butene [For the synthesis method, please refer to the invention patent applied by the applicant: 202010177342.6 Example 1] instead of 1-bromo-3-methyl-2-butene, it is convenient to prepare Obtained deuterated pentazocine prodrug (compound 2) 2.3g, HPLC content 99.1%.
[0085] GC-MS (m / z): 415.6.
Embodiment 3
[0086] The synthesis of embodiment 3 pentazocine prodrug (compound 1)
[0087] 1, Synthesis of 2-acetyl-2-methyl-butyrolactone (intermediate 1)
[0088] N 2 Protection, 4L of anhydrous dichloromethane solution of 2-acetylbutyrolactone (510g, 4mol), was added dropwise into [Me 2 Cl][Al(OTeF 5 ) 4 ] (8mol) solution, stirred at room temperature overnight, filtered, the solid was washed with an appropriate amount of dichloromethane, and concentrated under reduced pressure to obtain 523 g of light yellow oil, with a yield of 91.8%.
[0089] 2. Synthesis of 5-chloro-3-methylpentan-2-one (intermediate 2)
[0090] 2-Acetyl-2-methyl-butyrolactone (520g, 3.4mol), concentrated hydrochloric acid (1.3L, 32%, 10.7mol), distilled water 1500ml, heated carefully until the gas ceased, and the reaction compound was distilled to collect 1400ml distillate , the residue was added to 900ml of distilled water, and 800ml of distillate was collected by distillation, the organic layer was separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com