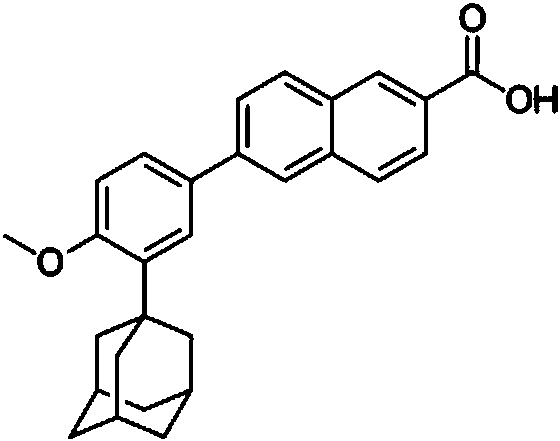
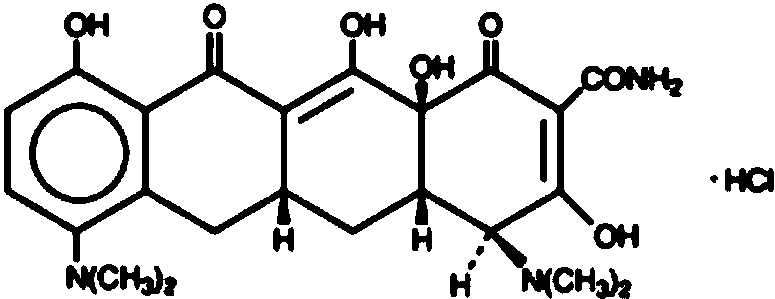
Topical pharmaceutical composition of adapalene and minocycline
A topical composition, minocycline technology, applied in the direction of drug combination, drug delivery, tetracycline active ingredients, etc., can solve problems such as instability, moisture, temperature and light sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
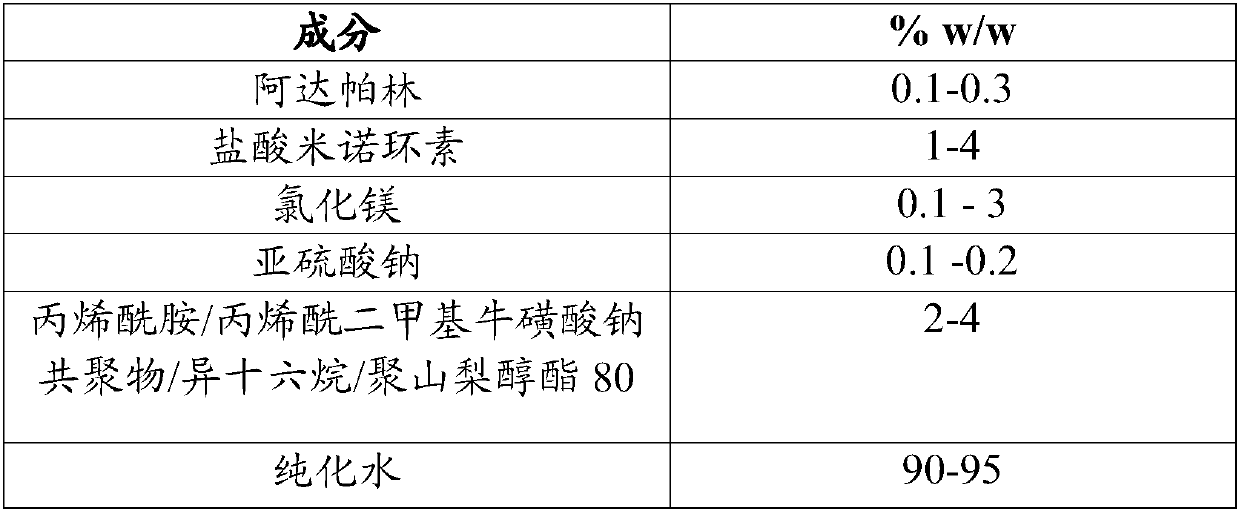
[0149] Embodiment 1: Adapalene / minocycline hydrochloride preparation
[0150] Table 1
[0151]
[0152] Preparation:
[0153] 1. Dissolve magnesium chloride in water with stirring at 500-1000 RPM for about 10 minutes until a clear solution is observed.
[0154] 2. Add sodium sulfite to the above solution with stirring at 500-1000 RPM for about 20 minutes to obtain a clear solution.
[0155] 3. Add minocycline hydrochloride to the solution formed in step 2 with stirring at 500-1000 RPM for 20-30 minutes.
[0156] 4. Add adapalene to the above mixture under stirring at 500-1000 RPM for 20-30 minutes, and homogenize the resulting mixture to obtain a uniform dispersion.
[0157] 5. Add acrylamide / sodium acryloyldimethyl taurate copolymer / isohexadecane / polysorbate 80 to the mixture formed in step (4) and homogenize for about 30 minutes to obtain a uniform gel glue dispersion.
Embodiment 2
[0158] Embodiment 2: Adapalene / minocycline hydrochloride preparation
[0159] Table 2
[0160]
[0161]
[0162] Preparation:
[0163] 1. Dissolve magnesium chloride in purified water with stirring at 500-1000 RPM for about 10 minutes until a clear solution is observed.
[0164] 2. Add sodium sulfite to the above solution with stirring at 500-1000 RPM for about 20 minutes to obtain a clear solution.
[0165] 3. Add minocycline hydrochloride to the solution formed in step (2) under stirring at 500-1000 RPM for 20-30 minutes to ensure complete dissolution of the drug in the solvent.
[0166] 4. Adapalene was dispersed in a solvent system comprising glycerin and propylene glycol under stirring at 500-1000 RPM for 20 minutes to obtain a homogeneous dispersion of API.
[0167] 5. The adapalene dispersion was then added to the solution mixture formed in step (3) with stirring at 500-1000 RPM for 20-30 minutes.
[0168] 6. Add acrylamide / sodium acryloyldimethyl taurate copo...
Embodiment 3
[0169] Embodiment 3: Adapalene / minocycline hydrochloride preparation
[0170] table 3
[0171] Element %w / w adapalene 0.1-0.3 Minocycline Hydrochloride 1-4 magnesium chloride 0.1-3 Sulfite 0.1-0.2 Propylene Glycol 4-10 glycerin 4-10 Sodium hyaluronate 1-2.5 purified water 70-85
[0172] Preparation:
[0173] 1. Dissolve magnesium chloride in purified water with stirring at 500-1000 RPM for about 10 minutes until a clear solution is observed.
[0174] 2. Add sodium sulfite to the above solution with stirring at 500-1000 RPM for about 20 minutes to obtain a clear solution.
[0175] 3. Add minocycline hydrochloride to the solution formed in step (2) with stirring at 500-1000 RPM for about 20-30 minutes to ensure complete dissolution of the drug in the solvent.
[0176] 4. Add sodium hyaluronate and disperse it into the solution mixture formed in step (3) under stirring at 1000-2000 RPM for about 30-60 minutes to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com