Method for synthesizing 4-methyl-2-benzothiazolehydrazine
A technology of benzothiazole and methylphenylthiourea, which is applied in the field of medicine, can solve the problems of high cost of starting raw materials, low product purity, and difficult product separation, achieving good social and economic benefits, and simple process operation , good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
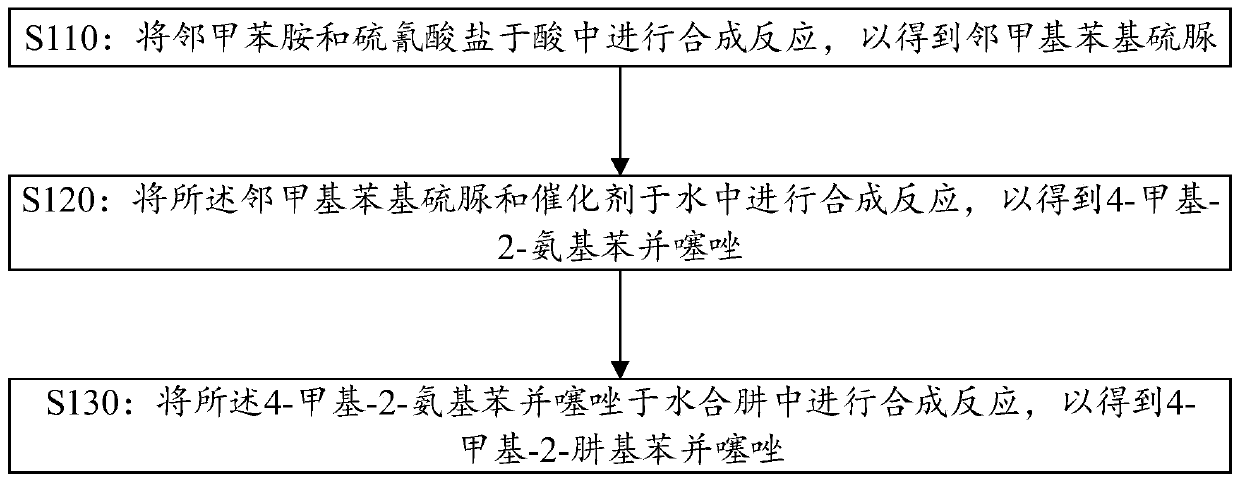
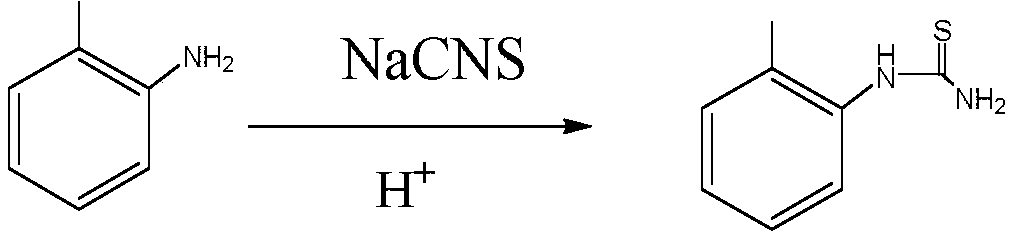
[0058] 1. Synthesis of o-methylphenylthiourea: put 1477Kg o-toluidine in a 5000L reactor, add 1830Kg of pre-configured 42% sulfuric acid, add 1336Kg of sodium thiocyanate under stirring, turn on steam heating, and reflux for 4 hours , cooling, discharging and centrifuging, washing the filter cake with water until neutral, and drying the filter cake to obtain 1410Kg of o-methylphenylthiourea.
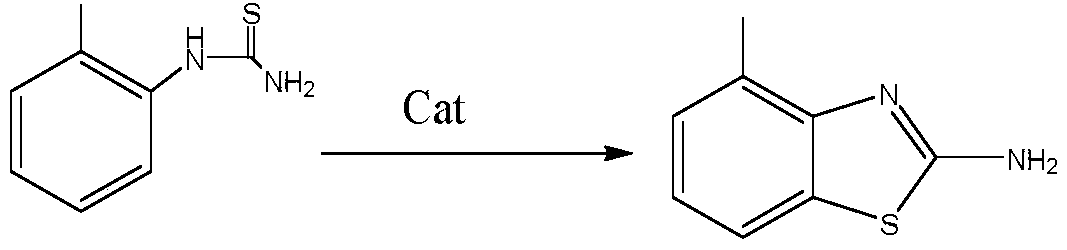
[0059] 2, the synthesis of 4-methyl-2-aminobenzothiazole: add 600Kg water in 3000L reactor, then the o-methylphenylthiourea obtained in the previous step reaction is put into reactor, add catalyst sodium bromide 28Kg, control The temperature is 25-30°C, react for 6 hours, cool, discharge and centrifuge, and dry the filter cake to obtain 1280Kg of 4-methyl-2-aminobenzothiazole.
[0060] 3. Synthesis of 4-methyl-2-hydrazinobenzothiazole: Add 40% hydrazine hydrate 1076Kg in a 3000L reactor, put the 4-methyl-2-aminobenzothiazole obtained in the previous step into the reactor, and stir Next,...
Embodiment 2
[0062] 1. Synthesis of o-methylphenylthiourea: put 1477Kg o-toluidine into 5000L reaction, add 2560Kg of pre-configured 30% sulfuric acid, add 1470Kg of sodium thiocyanate under stirring, turn on steam heating, reflux reaction for 4 hours, Cool, discharge and centrifuge, wash the filter cake with water until neutral, and dry the filter cake to obtain 1420Kg of o-methylphenylthiourea.
[0063] 2, the synthesis of 4-methyl-2-aminobenzothiazole: add 800Kg water in 3000L reactor, then the o-methylphenylthiourea gained in the previous step reaction is dropped into reactor, add catalyst calcium bromide 29Kg, control The temperature is 20-30 degrees, react for 6 hours, cool, discharge and centrifuge, and dry the filter cake to obtain 1210Kg of 4-methyl-2-aminobenzothiazole.
[0064] 3. Synthesis of 4-methyl-2-hydrazinobenzothiazole: Add 80% hydrazine hydrate 1050Kg in a 3000L reactor, put the 4-methyl-2-aminobenzothiazole obtained in the previous step into the reactor, and stir , tu...
Embodiment 3
[0066] 1. Synthesis of o-methylphenylthiourea: put 1605Kg o-toluidine into 5000L reaction, add 2500Kg of pre-configured 30% sulfuric acid, add 1337Kg of sodium thiocyanate under stirring, turn on steam heating, reflux reaction for 6 hours, Cool, discharge and centrifuge, wash the filter cake with water until neutral, and dry the filter cake to obtain 2270Kg of o-methylphenylthiourea.
[0067] 2, the synthesis of 4-methyl-2-aminobenzothiazole: add 1200Kg water in 5000L reactor, then put the o-methylphenylthiourea gained in last step reaction into reactor, add catalyst sodium bromide 50Kg, control The temperature is 40-50 degrees, react for 6 hours, cool, discharge and centrifuge, and dry the filter cake to obtain 2050Kg of 4-methyl-2-aminobenzothiazole.
[0068] 3. Synthesis of 4-methyl-2-hydrazinobenzothiazole: Add 80% hydrazine hydrate 1570Kg in a 5000L reactor, put 4-methyl-2-aminobenzothiazole obtained in the previous step into the reactor, and stir Next, turn on the heati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com