A diamine compound containing bis(diphenylamine)-tetraphenylethylene structure and its preparation method, a polyamide and its preparation method
An amine compound and tetraphenylethylene technology, which is applied in the field of electronically controlled fluorescence, can solve problems such as limited application, and achieve the effects of short response time, improved ion doping rate, and good cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The present invention also provides the preparation method of the diamine compound containing bis(diphenylamine)-tetraphenylethylene structure described in the above technical scheme, comprising the following steps:
[0051] (1) In a protective atmosphere, 4-halogenated nitrobenzene, Carry out nucleophilic substitution reaction with basic catalyst in polar organic solvent to obtain diphenylamine derivatives;
[0052] said where R is -CH 3 、-OCH 3 、-OCH 2 CH 3 , -N(CH 3 ) 2 or -C(CH 3 ) 3 ;
[0053] The diphenylamine derivative has a structure shown in formula II:
[0054]
[0055] (2) In a protective atmosphere, the diphenylamine derivatives obtained in the step (1), 1,1-diphenyl-2,2-bis(4-halogenated phenyl)ethene derivatives and catalysts in aromatic hydrocarbon Carry out coupling reaction in the solvent, obtain dinitro compound, described dinitro compound has the structure shown in formula III:
[0056]
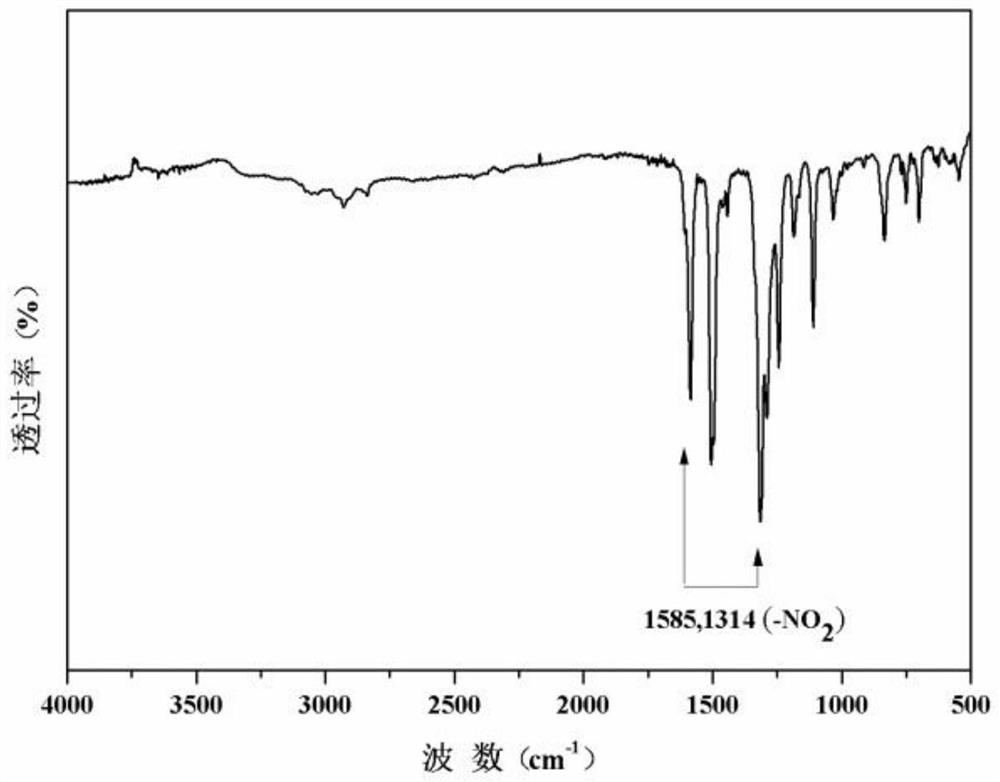
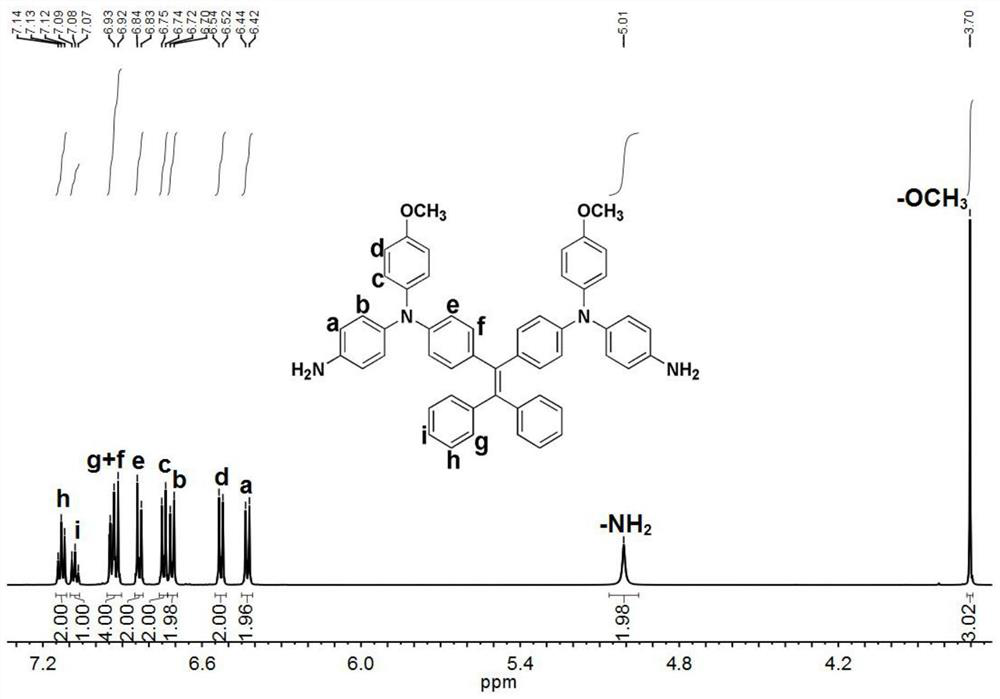
[0057] (3) carry out reduction reaction wi...
Embodiment 1
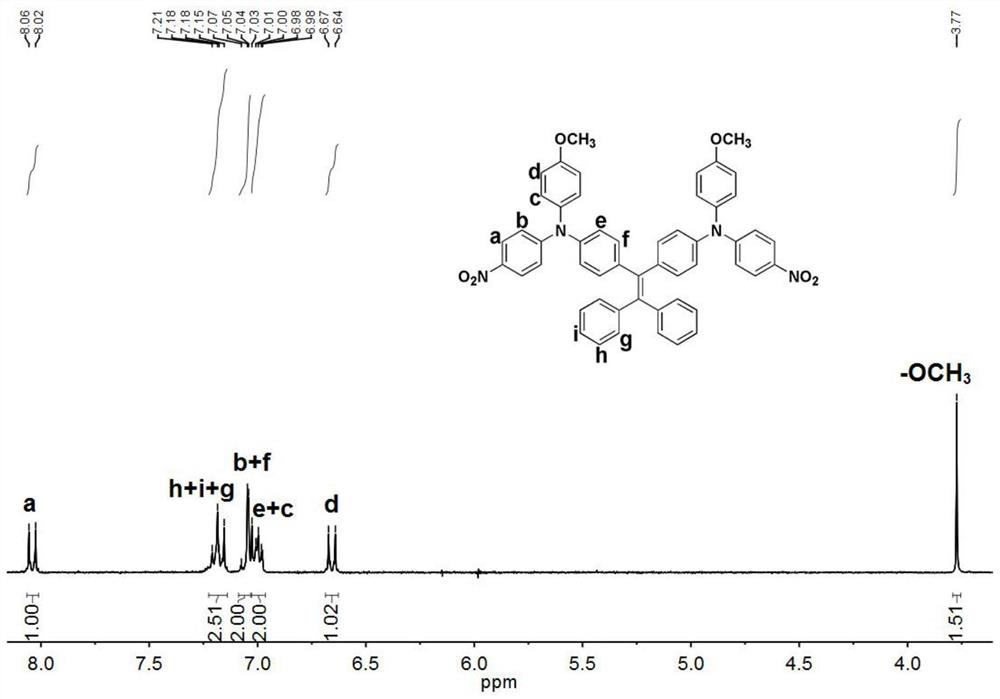
[0088] Mix 40g (324.8mmoL) of p-methoxyaniline, 35.2540g (249.9mmoL) of p-fluoronitrobenzene, 32.8672g (324.8mmoL) of triethylamine and 273mL of dimethyl sulfoxide, stir and nitrogen Under protected conditions, react at 85°C for 36 hours; after the reaction is completed, cool to room temperature, discharge the material in ice water, stir well, and filter to obtain the crude product; mix the crude product with N,N-dimethylacetamide and ethanol (Volume ratio 1:2) was prepared into a hot saturated solution, the temperature of the hot saturated solution was 90°C, then naturally cooled to room temperature, filtered, and the resulting solid was vacuum-dried at 80°C to obtain 50.1 g of orange 4-nitro- 4'-methoxyl-diphenylamine, yield rate is 82.1%, the structural formula of 4-nitro-4'-methoxyl-diphenylamine is as follows:
[0089]
[0090] 40g (163.8mmoL) of 4-nitro-4'-methoxy-diphenylamine, 34.65g (70.68mmoL) of 1,1-diphenyl-2,2-bis(4-bromophenyl)ethylene, Mix 36.19g (565.4mmoL)...
Embodiment 2
[0101] 45g (301.5mmoL) of 4-tert-butylaniline, 37.4966g (251.25mmoL) of p-fluoronitrobenzene, 30.5088g (301.5mmoL) of triethylamine and 308mL of dimethyl sulfoxide were mixed, stirring and Under the condition of nitrogen protection, react at 85°C for 36h; after the reaction is completed, cool to room temperature, discharge the material in ice water, stir well, and filter to obtain the crude product; the crude product is mixed with N,N-dimethylacetamide and Ethanol (volume ratio 1:2) was prepared into a hot saturated solution, the temperature of the hot saturated solution was 90°C, then naturally cooled to room temperature, filtered, and the resulting solid was vacuum-dried at 80°C to obtain 58.0 g of orange 4-nitro -4'-tert-butyl-diphenylamine, the productive rate is 85.4%, the structural formula of 4-nitro-4'-tert-butyl-diphenylamine is as follows:
[0102]
[0103] 40g (147.0mmoL) of 4-nitro-4'-tert-butyl-diphenylamine, 32.05g (65.37mmoL) of 1,1-diphenyl-2,2-bis(4-bromoph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com