Virus sample preserving fluid for viral nucleic acid clinical detection and use method thereof
A virus nucleic acid and preservation solution technology, which is applied in the field of virus sample preservation solution and kits, can solve the problems of false negative detection and insufficient number of new coronavirus nucleic acids, so as to ensure the integrity and quantity, improve the detection accuracy, and ensure the free of charge. affected by degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
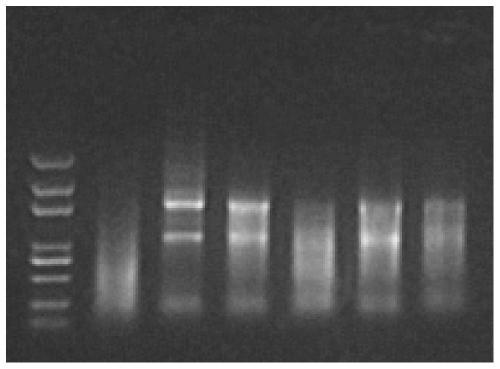
[0052] Example 1: The effect of inactivation treatment at 56°C on the quality of intracellular RNA in samples preserved with Trizol
[0053] 72hpf juveniles were collected for extraction of total RNA, 15 fish in each group. After the water was blotted dry, total RNA was obtained by TriZol method.
[0054] 1) Divide into 3 experimental groups in total, and each group has 3 repetitions: in the first group, add 500 μl Trizol to each repetition group, put it in a 56-degree water bath, and after 30 minutes in the water bath, use a homogenizer to homogenize (with No agglomeration is the best); in group 2, add 500 μl Trizol to each repeated group, put it in a refrigerator at 4 degrees, and after standing for 30 minutes, use a homogenizer to homogenize (no agglomeration is the best); group 3 Add 500 μl 1xPBS to each repetition group, put it in a 56-degree water bath, and after 30 minutes of water bath, blot the PBS solution dry, add 500 μl Trizol, and use a homogenizer to homogenize (...
Embodiment 2
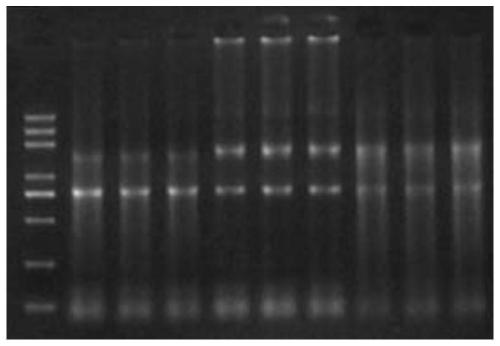
[0066] Example 2: The impact of samples stored in different preservation solutions on the quality of intracellular RNA after inactivation at 56°C
[0067] Collect 1800 μl of human cell solution per tube, centrifuge at 1500 g for 10 minutes, discard the supernatant, keep the precipitate, collect 6 tubes in total, and use the TriZol method to obtain total RNA.
[0068] 1) The 6 tubes of cells were equally divided into 3 experimental groups: the first group was added with 500ul RNA preservation solution 1 (100mM Tris (PH7.5), 12.5mM EDTA, 150mM Nacl, 250ug / ml proteinase K), and one tube was placed in 56 put the other tube in a 4-degree refrigerator and let it stand for 30 minutes; the second group was to add 500 μl RNA preservation solution 2 (0.5% SDS, 100mM Nacl, 10mM Tris-Hcl (PH8.0), 50mM EDTA, 100ug / ml proteinase K), put one tube in a 56°C water bath for 30 minutes, and put the other tube in a 4°C refrigerator for 30 minutes; the third group was to add 500μl RNA preservation...
Embodiment 3
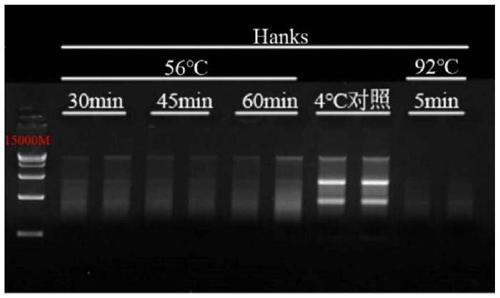
[0080] Example 3: Effects of inactivation treatment at 56°C on the quality of intracellular RNA in samples stored in Hanks solution and Vazyme virus sample preservation solution
[0081]HEK-293 cells (self-cultivation), porcine epidemic diarrhea virus (Procine epidemic diarrhea virus, PEDV) (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences) and lambda DNA (Takara #3010) were used as experimental samples.
[0082]
[0083] Fully dissolve PEDV with 10mL PBS, aliquot and store at -20°C until use. Configure the mixing tube according to the above table, and divide it into 1.5mL / tube, and place it in a refrigerator at 4°C, a water bath at 56°C, and a water bath at 92°C for different treatment times.
[0084] Novizyme Virus Sample Preservation Solution (Vazyme#R503) was pre-dispensed with 200 μL of absolute ethanol into a 1.5 mL RNase-free centrifuge tube, then added 500 μL of the sample preserved in the virus sample preservation solution, and vortexe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com