Disposable Injectable Cervical Dilation Rod
An injection-type, disposable technology, applied in the field of medical equipment, can solve the problems of increasing the suffering of patients, induced abortion syndrome, intrauterine infection, etc., and achieve the effect of improving water absorption, good water retention and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
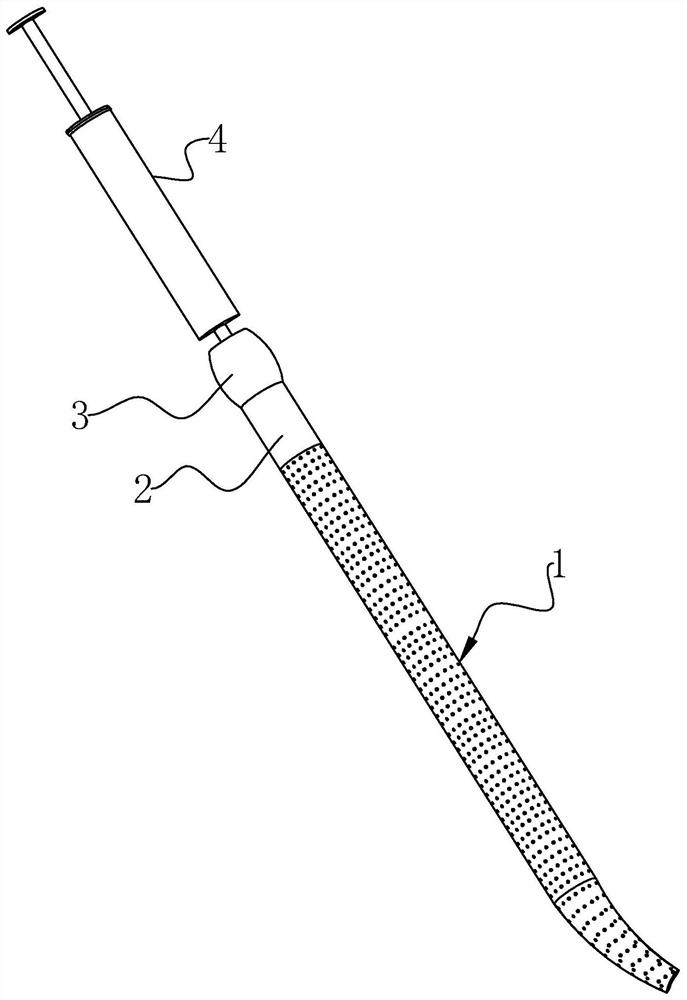
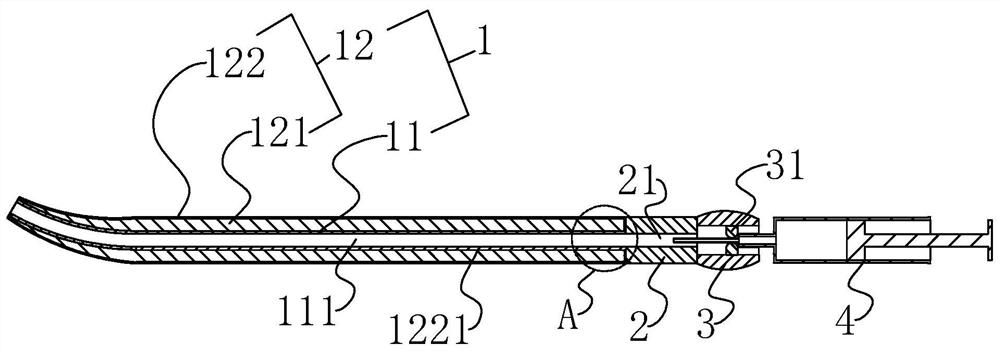
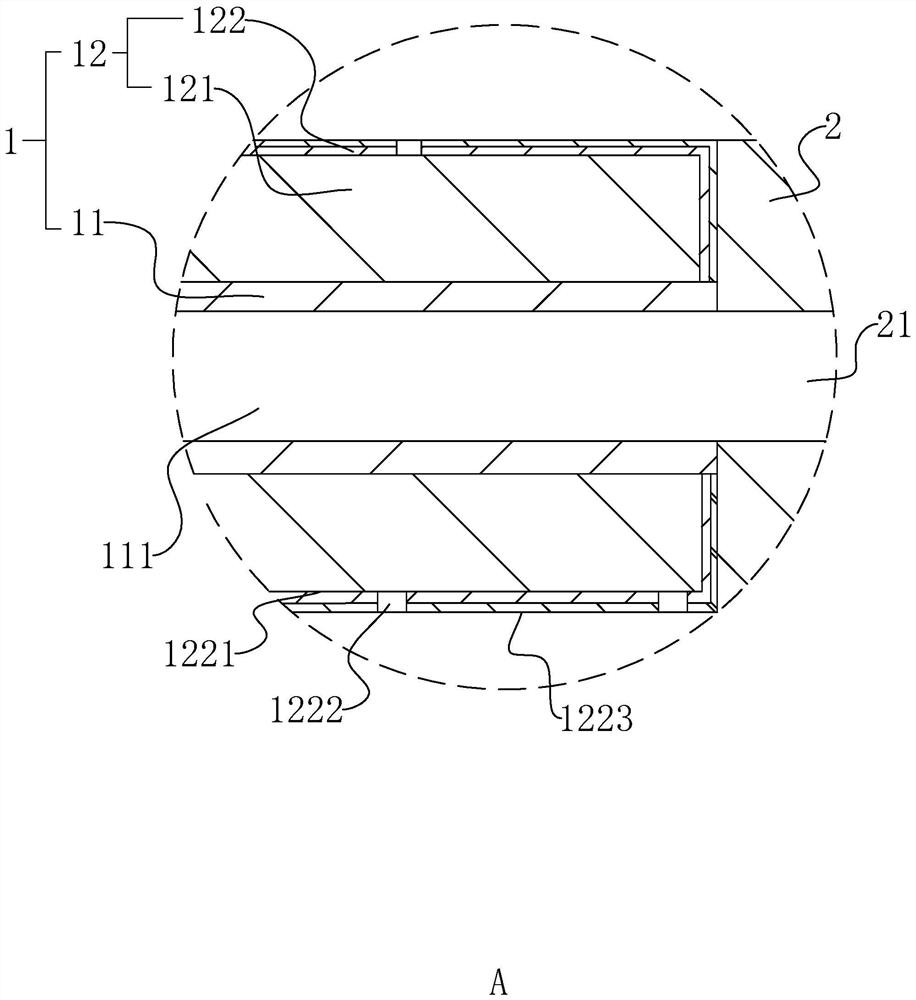
[0041] The invention discloses a disposable injection type cervical dilation rod, such as figure 1 and figure 2 As shown, it includes a dilation head 1, a liquid guide rod 2 and an injection joint 3. The liquid guide rod 2 is provided with a liquid guide hole groove 21 along the length direction, and the injection joint 3 and the dilation head 1 are respectively arranged in Both ends of the catheter rod 2, at the same time, in this embodiment, the end of the dilation head 1 away from the catheter rod 2 is distorted at 25° and gradually becomes thinner; the dilation head 1 includes a hydrophobic liquid catheter 11 and a pro- The water expansion head 12, the hydrophobic liquid guide head 11 is arranged at the end of the liquid guide rod 2 away from the injection joint 3, and the hydrophobic liquid guide head 11 is provided with a drainage groove 111 which communicates with the liquid guide hole groove 21 along the length direction. The hydrophilic expansion head 12 is disposed...
Embodiment 2
[0044] The raw materials of the disposable injectable cervical dilation rod, the liquid guide rod and the injection joint all include the following components by weight: 23 parts of polyethylene, 20 parts of polypropylene, 36 parts of oxidized polyethylene wax, and 16 parts of titanium powder.
[0045] The raw material of the hydrophobic liquid-guiding head includes the following components by weight: 21 parts of polyethylene, 20 parts of polypropylene, 16 parts of oxidized polyethylene wax, and 18 parts of polydimethylsiloxane.
[0046] The raw materials for the expansion inner material include the following components in parts by weight: 30 parts of croscarmellose sodium, 21 parts of potassium polyacrylate, 22 parts of polyethylene oxide, and 3 parts of gelatin sponge.
[0047] The raw material of the elastic protective film includes the following components by weight: 26 parts of natural latex, 12 parts of linear low density polyethylene, and 2 parts of soybean lecithin.
...
Embodiment 3
[0050] The raw materials of the disposable injectable cervical dilation rod, the liquid guide rod and the injection joint all include the following components by weight: 25 parts of polyethylene, 26 parts of polypropylene, 31 parts of oxidized polyethylene wax, and 15 parts of titanium powder.
[0051] The raw material of the hydrophobic liquid-guiding head includes the following components by weight: 20 parts of polyethylene, 18 parts of polypropylene, 17 parts of oxidized polyethylene wax, and 19 parts of polydimethylsiloxane.
[0052] The raw materials for the expansion inner material include the following components in parts by weight: 32 parts of croscarmellose sodium, 25 parts of potassium polyacrylate, 19 parts of polyethylene oxide, and 20 parts of gelatin sponge.
[0053] The raw material of the elastic protective film includes the following components by weight: 23 parts of natural latex, 10 parts of linear low density polyethylene, and 3 parts of soybean lecithin.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com