Hydroxylated fatty acid homopolymer and production method thereof
一种氢氧化脂肪酸、制造方法的技术,应用在氢氧化脂肪酸的均聚物领域,能够解决比例变少、未有所报告等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0280]
[0281] 0.3 mL of milliQ water was added to 184 mg of 10-hydroxy-cis-12-octadecenoic acid, and ultrasonic treatment was performed for 20 seconds. 11 mg of lipase AY "Amano" 30SD was added, stirred at 37° C. and 130 rpm for 24 hours, and reacted. Utilize the Bligh-Dyer method to extract the liquid after the reaction, and utilize silica gel chromatography (developing solvent hexane:diethyl ether:acetic acid=40:60:1) to carry out separation and purification, obtain the 2 polymer (9.4mg) of HYA , a 3-mer of HYA (4.7 mg) and a 4-mer of HYA (7.9 mg).
[0282] 2-mer of HYA
[0283] Rf value = 0.30 by silica gel chromatography (developing solvent: hexane: diethyl ether: acetic acid = 40:60:1).
[0284]
[0285] 3-mer of HYA
[0286] The Rf value based on silica gel chromatography (developing solvent: hexane: diethyl ether: acetic acid = 40:60:1) = 0.38.
[0287]
[0288] 4-mer of HYA
[0289] The Rf value by silica gel chromatography (developing solvent: hexane: d...
Embodiment 2
[0293]
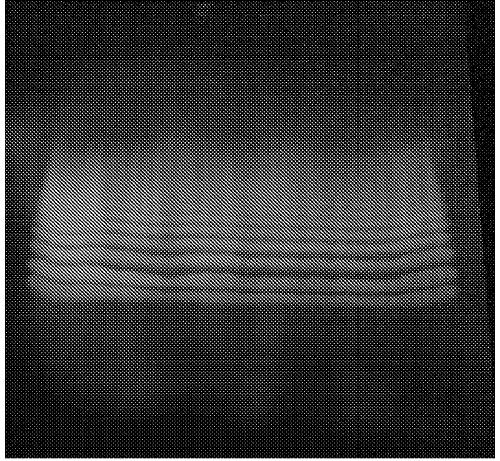
[0294] 0.3 mL of milliQ water was added to 184 mg of 10-hydroxy-cis-12-octadecenoic acid, and ultrasonic treatment was performed for 20 seconds. Then, 11 mg of lipase AY "Amano" 30SD was added, and it stirred and reacted at 37 degreeC and 130 rpm for 24 hours. Only oil and fat components were extracted from the reacted liquid by the Bligh-Dyer method, and the liquid concentrated by a rotary evaporator was subjected to thin-layer chromatography (TLC Silica gel 60 F254; developing solvent: hexane: diethyl ether: Acetic acid=40:60:1). The results of thin layer chromatography (365nm ultraviolet irradiation) are shown in figure 1 .
[0295] according to figure 1 It is possible to confirm the point where HYA aggregated. From the number of points, it was confirmed that HYA formed 2-10 polymers.
[0296] The Rf value of each point is shown below.
[0297] 2-mer of HYA: 0.30
[0298] 3-mer of HYA: 0.38
[0299] 4-mer of HYA: 0.42
[0300] 5-mer of HYA: 0.46
[0301...
Embodiment 3
[0307]
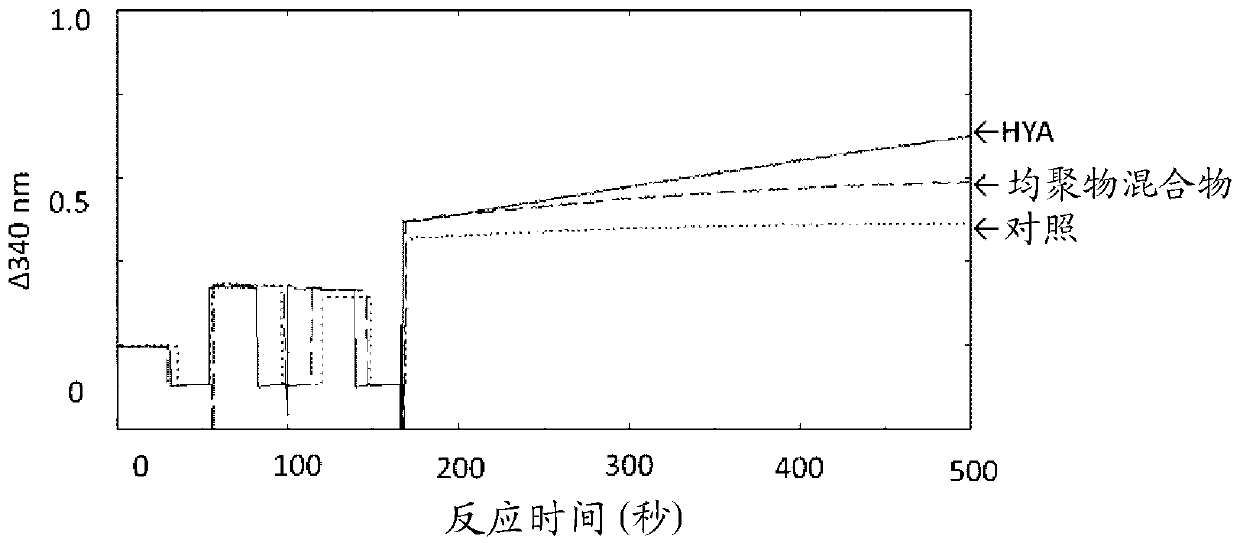
[0308] The oxidation stability of the reaction extract (homopolymer mixture) obtained in Example 1 was compared with that of HYA. Oxidation reactions decompose NAD in the presence of fatty acid hydroxide dehydrogenase (CLA-DH) + Implemented as an electron acceptor (S. Kishino et al., Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA, 110(44), 17808-17813, 2013).
[0309] The reaction solution (3 mL) contained 150 μL of a cell-free extract of Escherichia coli expressing CLA-DH as a hydrogenated fatty acid dehydrogenase (from 50 mg of E. coli wet cells), 4 mM NAD + , 150 μL of ethanol and 0.48 mM (0.143 mg / mL) of HYA as a substrate or an equivalent (0.143 mg / mL) of a homopolymer mixture, the reaction is initiated by the addition of hydrogenated fatty acid dehydrogenase and carried out at 37 °C About 6 minutes. It should be noted that, as a control, no matrix was added. During the oxidation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com