Pharmaceutical composition for treating traumatic brain injury and preparation thereof
A composition and traumatic technology, applied in the field of medicine, can solve problems such as the record of the treatment effect of traumatic brain injury TBI and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The following non-limiting examples are provided to further illustrate the invention.
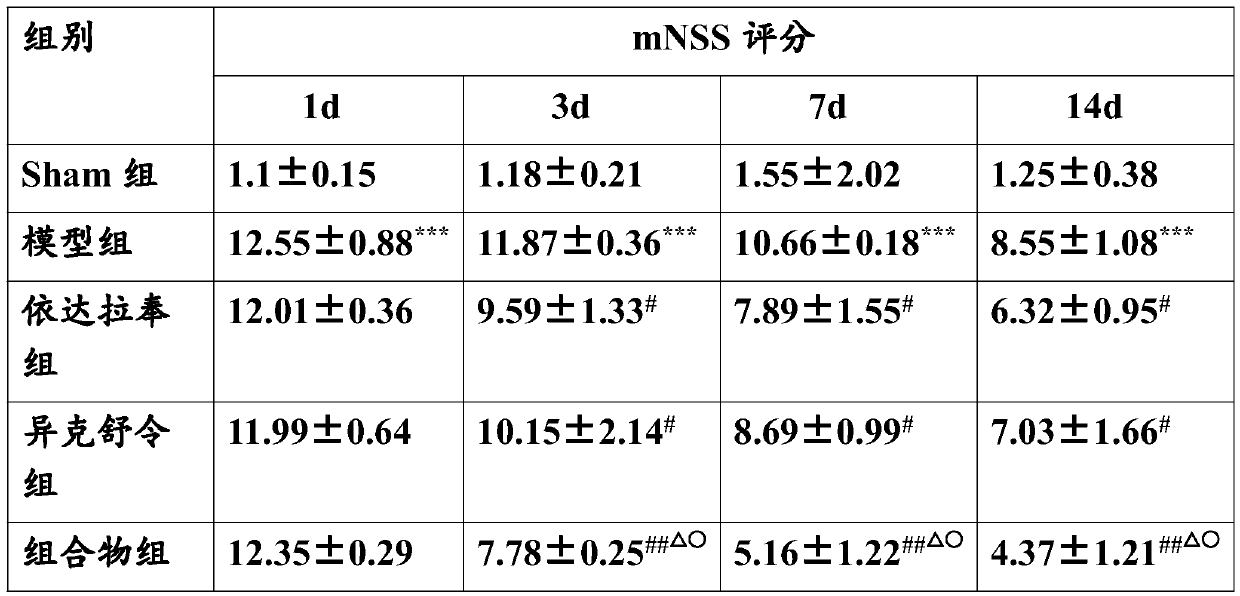
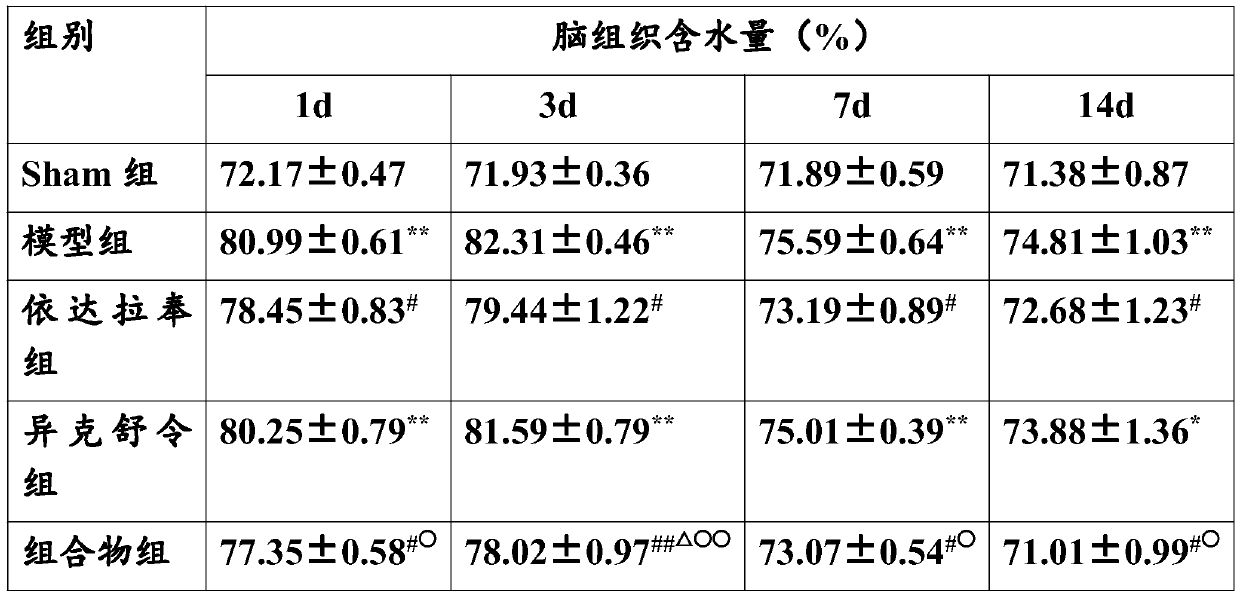
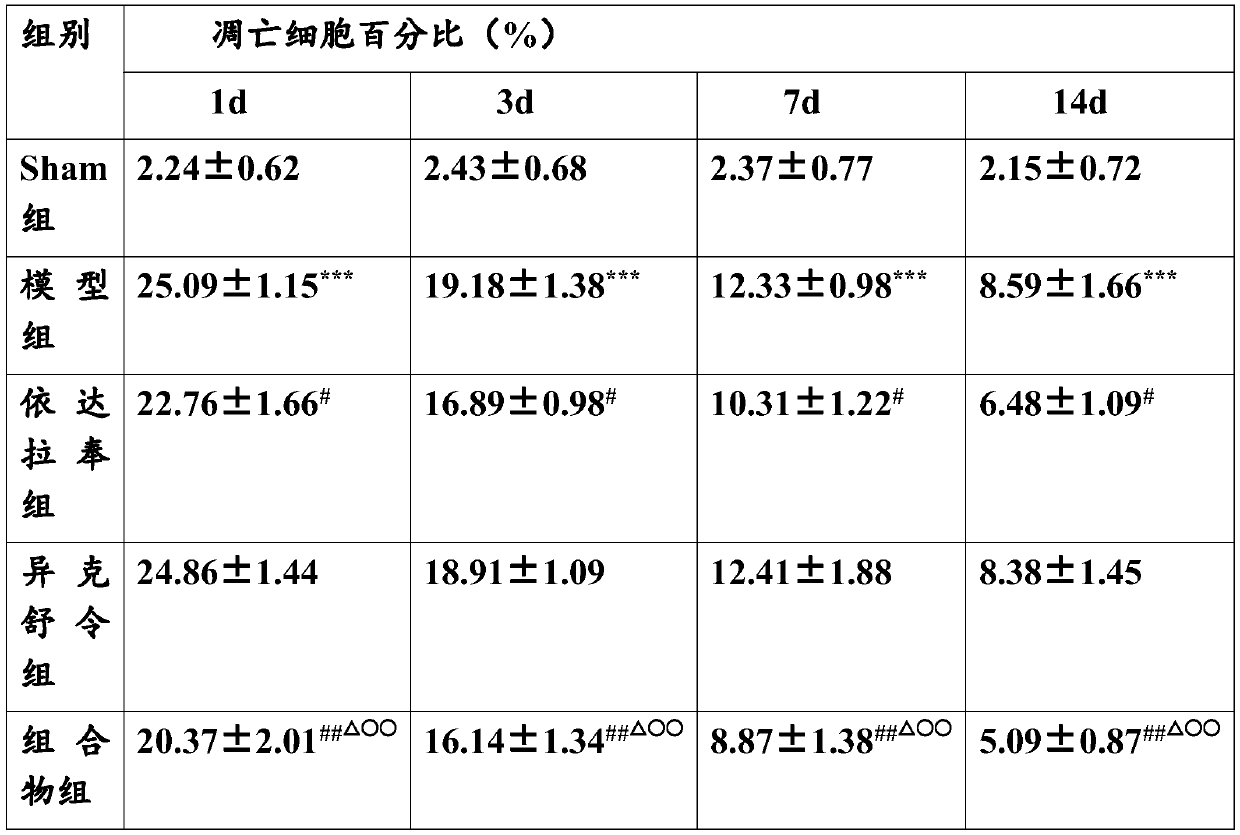
[0022] Embodiment The effect of combined application of edaravone and isoxuling on TBI rats in vivo animal experiments
[0023] 1. Experimental materials and model preparation
[0024] 1. Materials
[0025] Adult male clean SD rats, weighing about 250g. TUNEL apoptosis detection kit; TNF-α, IL-6, IL-1α, IL-1β enzyme-linked immunosorbent assay (ELISA) kit; Edaravone (Edaravone injection: 5ml (10mg) / branch, Nanjing Simcere Dongyuan Pharmaceutical Co., Ltd.); Isoxalin (Isotalin hydrochloride, sigma company).
[0026] 2. Experimental animals and grouping
[0027] Healthy male SD rats, weighing about 250g, 50 were randomly divided into five groups, 10 in each group, grouped as follows:
[0028] The first group: sham operation group (Sham group)
[0029] The second group: TBI group (model control group)
[0030] The third group: Edaravone 3mg / kg
[0031] The fourth group: 1 mg / kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com