Industrial synthesis method of dichocrocis punctiferalis sex pheromone
A synthesis method and pheromone technology, applied in the direction of organic chemistry methods, chemical instruments and methods, introduction of halogen preparation, etc., can solve the problems of harsh reaction conditions, inflammable and explosive, unsuitable for large-scale production, etc., to avoid easy Inflammable and explosive safety hazards, the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
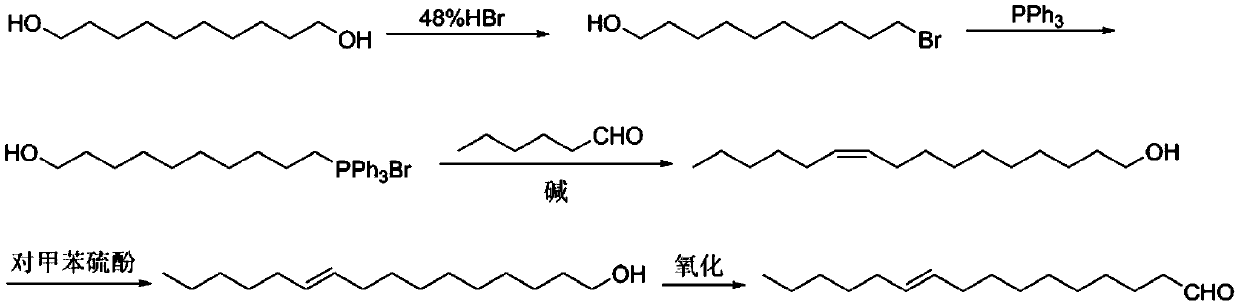
[0024] Synthesis of 10-Bromo-1-Decanol
[0025] Add 1,10-decanediol (321g, 1.712mol), toluene (1000ml), 48% hydrobromic acid (231ml, 2.054mol, 1.2eq) into a 2L three-necked flask, heat to 110°C, and reflux for 24 hours Add 48% hydrobromic acid (84ml, 0.753mol, 0.44eq) and continue heating to reflux for 24 hours. A small amount of raw material remains as detected by gas chromatography. Cool to room temperature and dilute with 500ml petroleum ether, separate the hydrobromic acid, wash the organic phase with saturated sodium bicarbonate (400ml×2) and saturated brine (400ml×2) successively, and dry over anhydrous sodium sulfate. Spin-dried and passed through the column to obtain 10-bromo-1-decanol (yield 84%).
Embodiment 2
[0027] Synthesis of 10-Hydroxydecyltriphenylphosphine Salt
[0028] Add 10-bromo-1-decanol (357g, 1.424mol), acetonitrile (1000ml), triphenylphosphine (409g, 1.566mol, 1.1eq) into a 2L three-necked flask, heat to 94°C and reflux for 48 hours. After the reaction, cool to room temperature and spin dry acetonitrile. Add 500ml of toluene, heat to reflux until homogeneous and stir for 15min, cool to room temperature, pour out the upper layer of toluene, repeat twice. Washing with diethyl ether can give white solid 10-hydroxydecyltriphenylphosphine salt with a yield of 88%.
Embodiment 3
[0030] Synthesis of 10-Hexadecen-1-ol
[0031] Add embodiment 2 gained quaternary phosphonium salt (655g, 1.278mol) in the there-necked flask of 3L mechanical stirring, tetrahydrofuran 1000ml, N 2 Open up, add potassium tert-butoxide (315g, 2.812mol, 2.2eq) in 3-4 times, control the reaction temperature below 20°C, and stir at room temperature for half an hour after the addition. Cool in an ice bath to 0°C, add a solution of n-hexanal (213g, 2.136mol, 1.2eq) in tetrahydrofuran (200ml), control the internal temperature not to exceed 5°C, after the addition is complete, react at 0°C for half an hour, then stir at room temperature for 1 hour. Add saturated ammonium chloride (500ml) to quench, separate the organic phase, extract the aqueous phase with ethyl acetate (500ml×2), wash the organic phase with brine (500ml×2), and dry over anhydrous sodium sulfate. The crude product was separated and purified by column chromatography (eluent petroleum ether / ethyl acetate=15:1~10:1) to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com