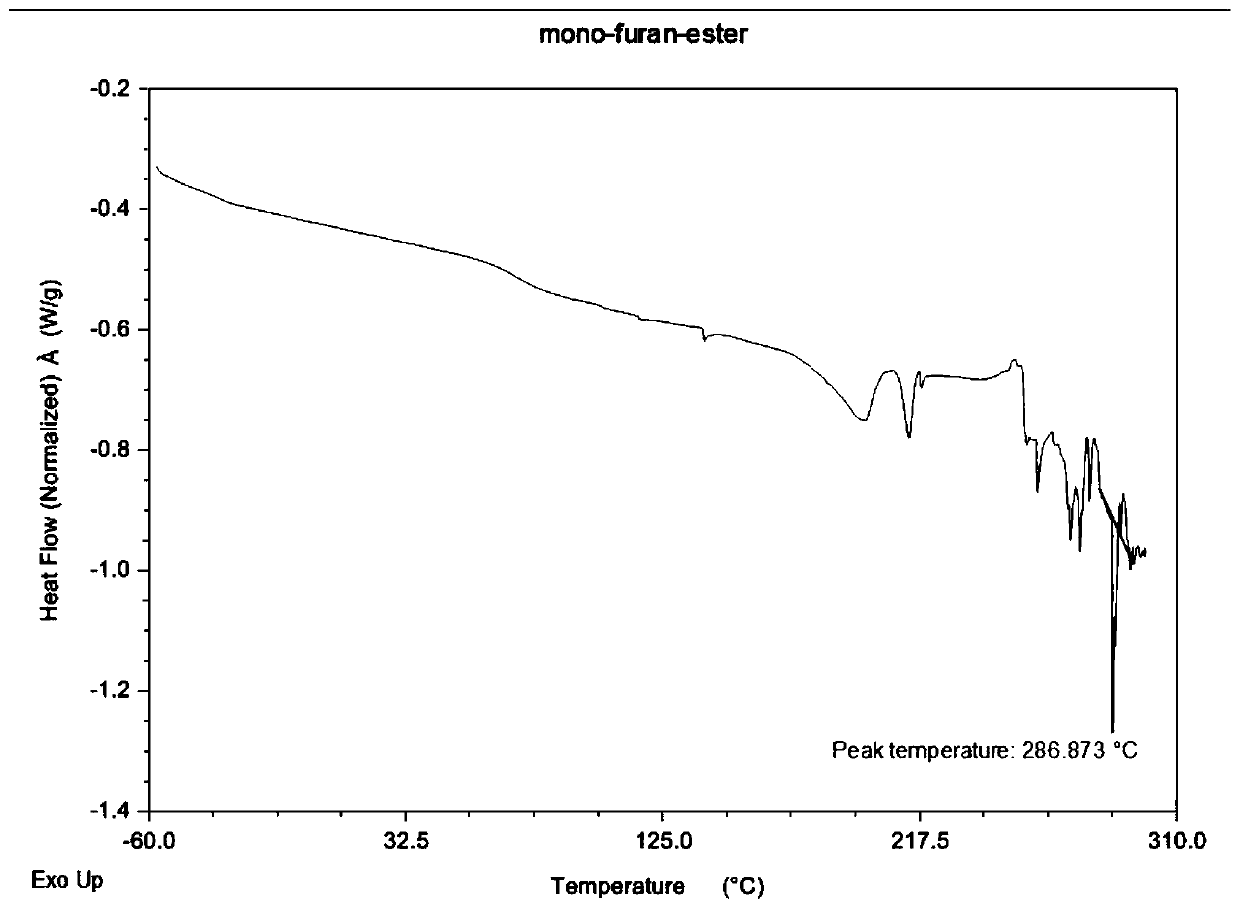
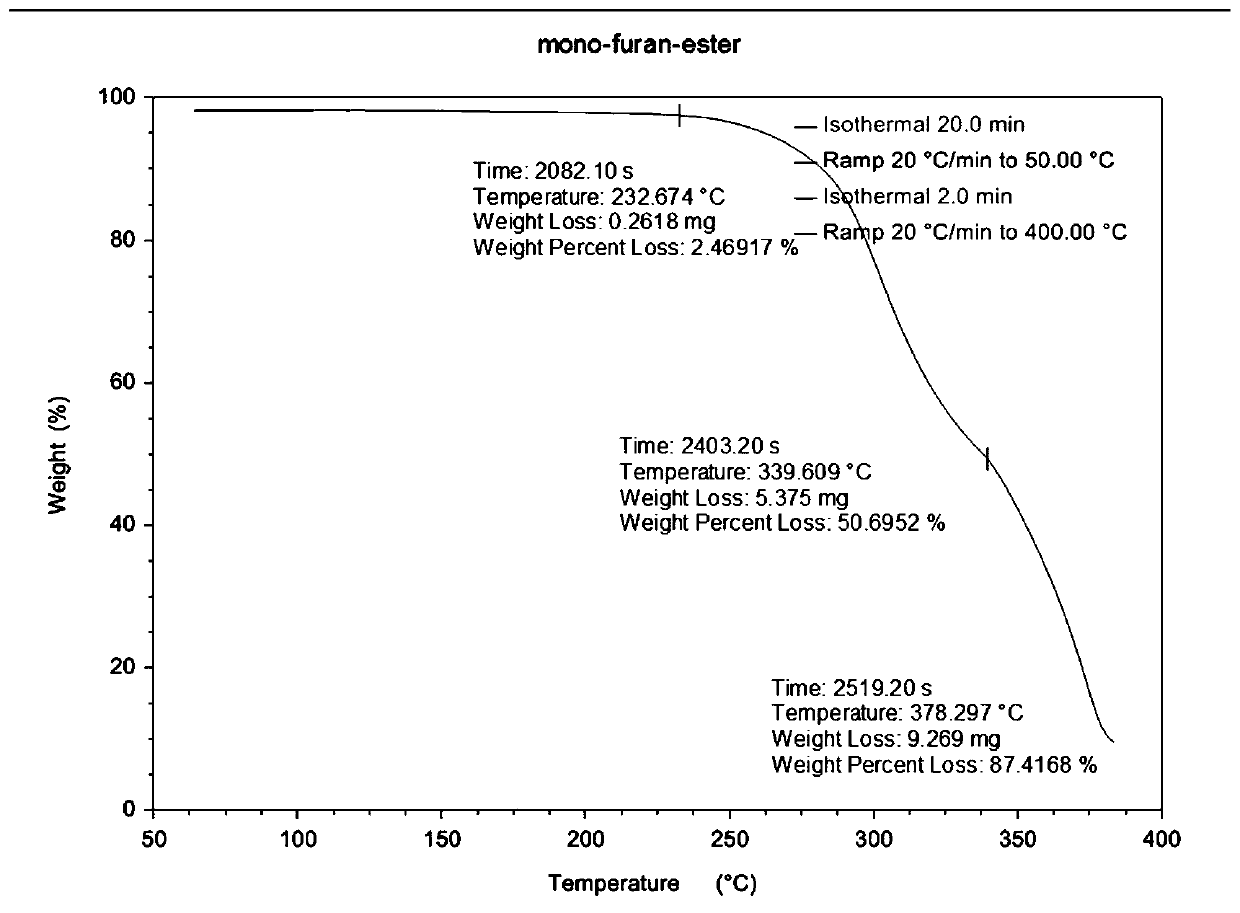
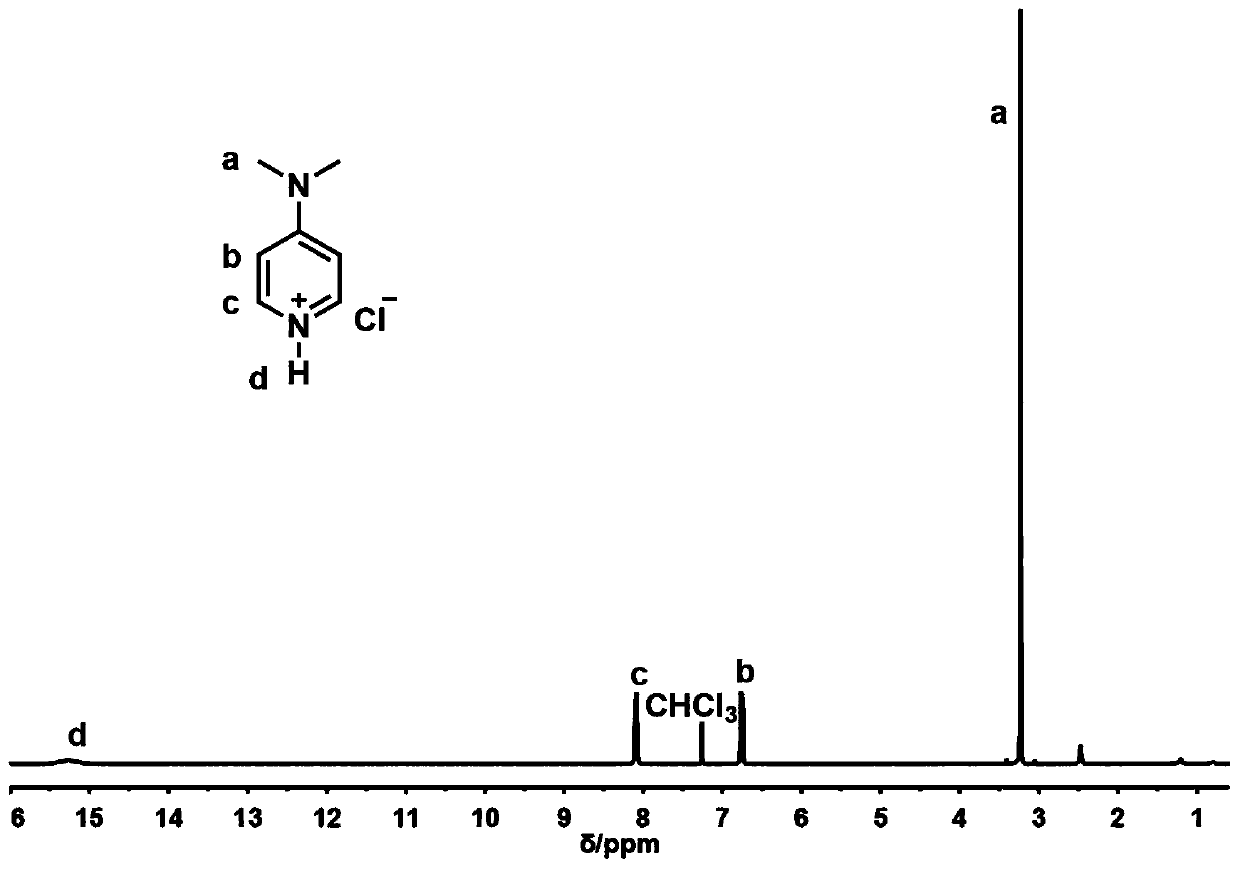
Preparation method of polyester containing furan ring
A furan ring and polyester technology, applied in the field of polyester preparation, can solve problems such as multiple side reactions, metal residues, complex synthesis, etc., and achieve the effects of accelerating the speed of the prepolymerization reaction, the synthesis method being simple, and making up for the interference of the reverse reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]In the three-necked flask, N2 was pumped three times, the temperature was slowly increased, and the stirring was continued. 90ml of 7 and 88ml of 10 (1:1) were added in a nitrogen-filled state, and 2.5g (1% wt) of 3 was added, and the reaction system was closed. , then heated to 180°C, and after 1.5 hours of reaction, nitrogen was flowed into the reaction system to make the small molecules evaporate with nitrogen. After the small molecules were evaporated, the system was sealed again, and then the temperature was raised to 230°C, and the reaction was continued. 3h, pumped air for 0.5h under 0.5mbar vacuum, stopped heating and stirring, cooled to room temperature with nitrogen, took out the product and washed the surface small molecules and oligomer residues with ethanol, and dried under vacuum to obtain white furan polymer. The ester solid was 216 g, and the yield was 88.0%.
Embodiment 2
[0062] In the three-necked flask, N2 was pumped for three times, the temperature was slowly increased, and the stirring was continued. 100ml of 8 and 166ml of 11 (1:1.2) were added under nitrogen, and 1.4g (0.5% wt) of 5 was added, and the reaction system was closed. , then heated to 170°C, after 1.5h of reaction, nitrogen was flowed into the reaction system to make small molecules evaporate with nitrogen gas, after the small molecules were evaporated, the system was sealed again, and then the temperature was raised to 250°C, and the reaction was continued for 2h , pumped air for 0.5h under 0.5mbar vacuum, stopped heating and stirring, ventilated and cooled to room temperature, took out the product and washed the surface small molecules and oligomer residues with ethanol, and dried under vacuum to obtain light yellow furan polyester solid 235g, 82.1% yield.
Embodiment 3
[0064] In the three-necked flask, N2 was pumped for three times, the temperature was slowly raised, and the reaction system was closed. , then heated to 180°C, after the reaction for 2h, the small molecules were evaporated, the system was sealed again, then the temperature was raised to 240°C, the reaction was continued for 2.5h, and the air was pumped for 0.5h under a vacuum of 0.5mbar, and the heating and stirring were stopped. The mixture was ventilated and cooled to room temperature, and the product was taken out and washed with ethanol to remove surface small molecules and oligomer residues. After drying in vacuum, 357 g of light yellow furan polyester solids were obtained with a yield of 84.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com