Ring-opening polymerization method of cyclic monomer
A cyclic monomer, ring-opening polymerization technology, applied in the field of green catalytic synthesis, can solve the problems of difficult preparation of metal catalysts and enzyme catalysts, inability to adapt to new requirements, unfavorable material stability, etc., and achieve narrow molecular weight distribution index, wide range, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
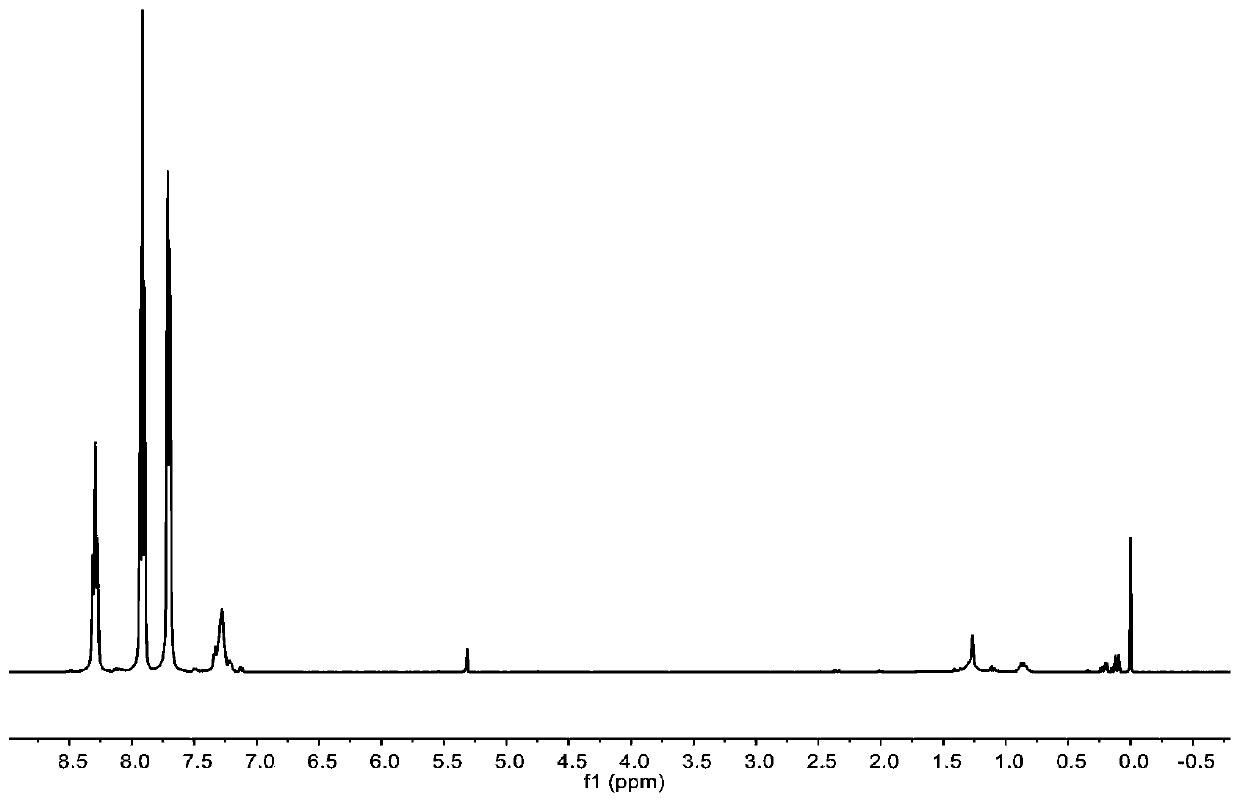
[0078] The preparation of carbocation Lewis acid 18, under the protection of anhydrous inert gas, 2.8g (15.4mmol) benzophenone, 23.1ml (in tetrahydrofuran, substance concentration is 1mol / L) phenylmagnesium bromide, use Anhydrous tetrahydrofuran was used as a solvent, and reacted at 60 degrees Celsius. After 2 hours of reaction, the reaction was complete, and 0.54ml (30mmol) of water was added to quench the reaction. After rotary evaporation, drying and recrystallization, 2.5g of triphenylmethanol was obtained. 62.5%. Dissolve 2.5g (9.6mmol) triphenylmethanol in anhydrous ether, cool down to 0°C, add 2.1ml (14.4mmol) tetrafluoroborate diethyl ether dicomplex dropwise to the reaction, and a yellow solid precipitates out immediately. After filtration and drying, 2.7g carbocation Lewis acid 18 was obtained, and its hydrogen spectrum structure was as follows: figure 1 shown.
[0079] In a 10mL polymerization tube, add δ-valerolactone (0.27ml, 3mmol), carbocation Lewis acid 18 (0...
Embodiment 2
[0081] Carbocation Lewis acid 18 was prepared as in Example 1.
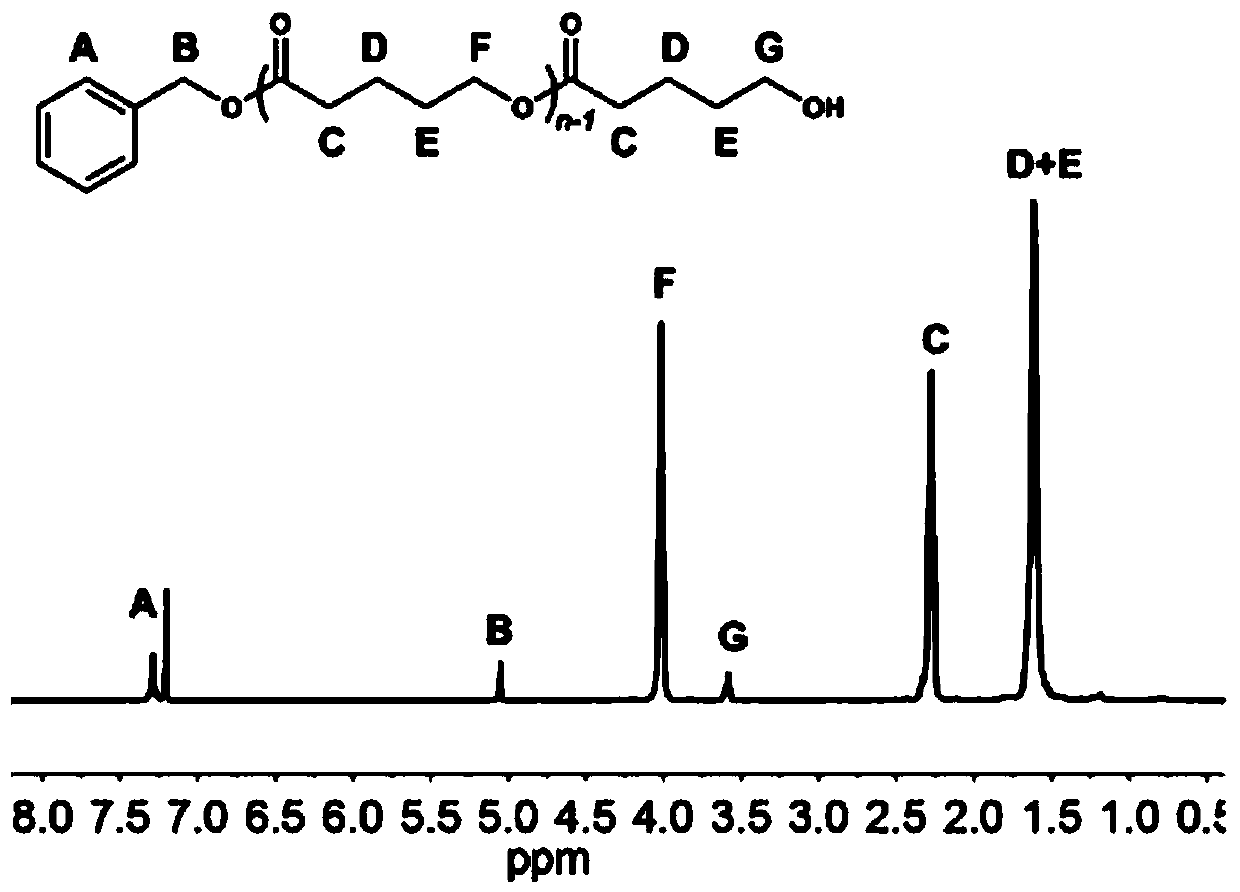
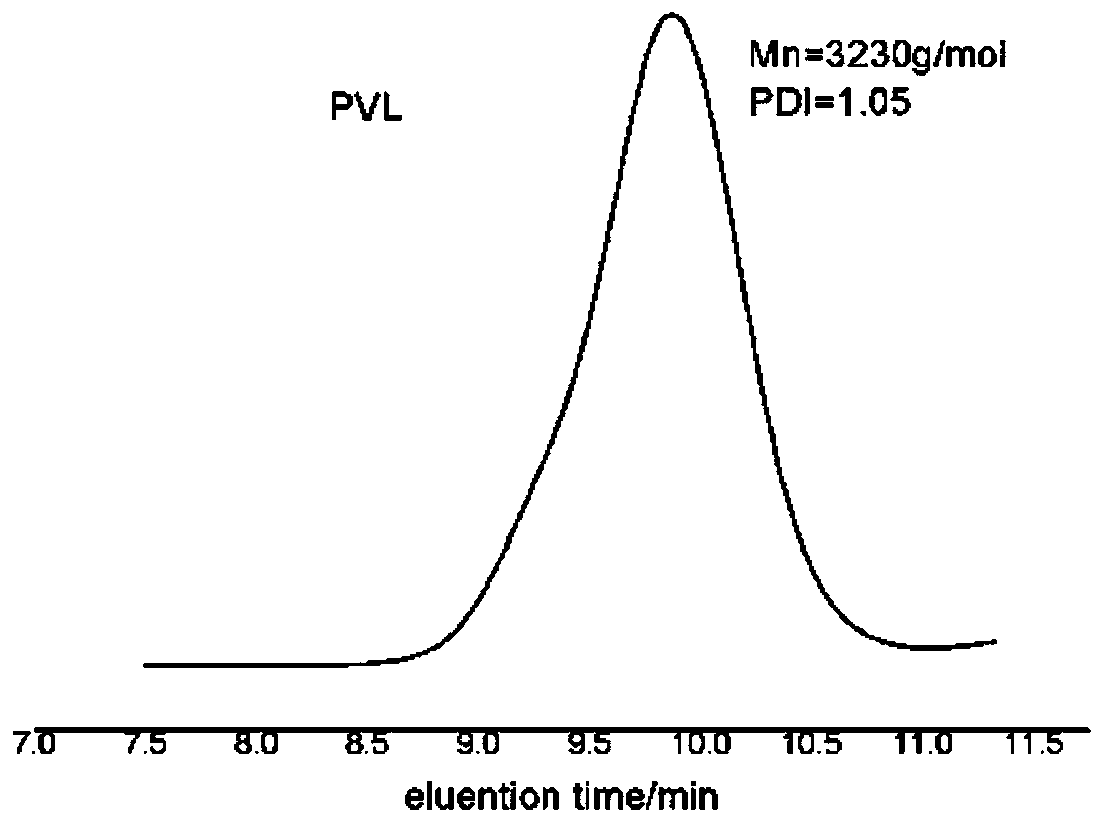
[0082] In a 10mL polymerization tube, add ε-caprolactone (0.33ml, 3mmol), carbocation Lewis acid 18 (0.033g, 0.1mmol), triphenylamine (0.0245g, 0.1mmol), benzyl alcohol (10.3μL, 0.1mmol), finally add 1mL of dichloromethane as a solvent, stir magnetically for 2.5 hours at 30°C, stop the reaction, slowly drop the resulting solution into cold methanol, a white polymer precipitates, centrifuge and vacuum dry to obtain snow-white Product 0.32g, transformation efficiency 95%, the number average molecular weight M of polycaprolactone n It is 3.4kg / mol, and the molecular weight distribution PDI is 1.10. The hydrogen spectrum of the product is as Figure 4 As shown, the size exclusion chromatography of the product is shown as Figure 5 shown.
Embodiment 3
[0084] Carbocation Lewis acid 18 was prepared as in Example 1.
[0085] In a 10 mL polymerization tube, add trimethylene carbonate (0.3063 g, 3 mmol), carbocation Lewis acid 18 (0.033 g, 0.1 mmol), triphenylamine (0.0245 g, 0.1 mmol), benzyl alcohol (10.3 μL, 0.1mmol), finally add 1mL of toluene as a solvent, stir magnetically for 2.5 hours at 60°C, stop the reaction, slowly drop the resulting solution into cold methanol, and a colorless and transparent oily substance precipitates out. After centrifugation and vacuum drying, a colorless and transparent viscous Thick thing 0.29g, transformation rate is 95%, the number average molecular weight M of polytrimethylene carbonate n It was 3.2 kg / mol, and the molecular weight distribution PDI was 1.15.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com