A method for synthesizing gamma-butyrolactone containing spirocyclic 1,3-indanedione structure using microchannel reaction device
A technology of microchannel reaction and nindione, which is applied in chemical instruments and methods, chemical/physical/physical chemical reactors, organic chemistry, etc., can solve the problems of cumbersome reaction steps, difficult treatment of acid wastewater, and low reaction efficiency. Achieve the effects of shortening the reaction time, easy control of the reaction process, and speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
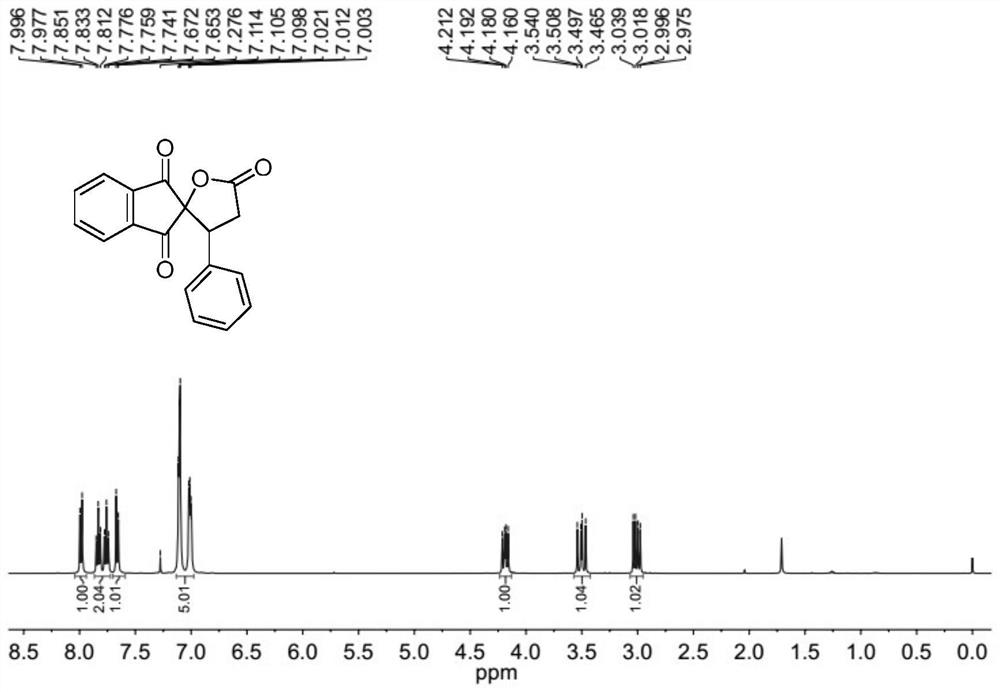
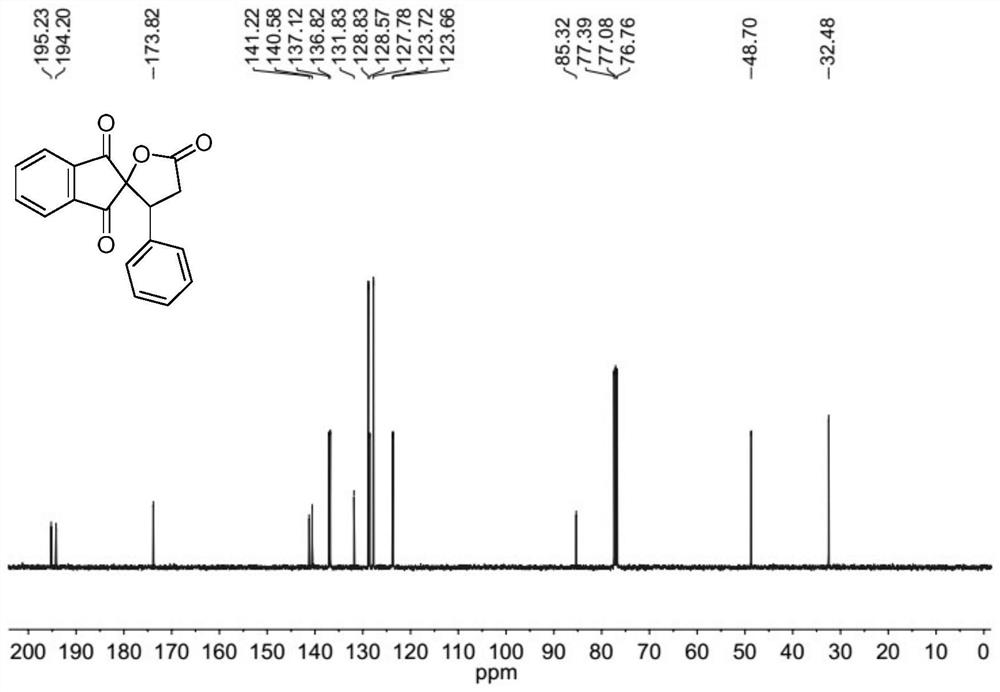
[0078] The molar ratio of 2-benzylidene indene-1,3-dione to cyclo(ethylene)isopropyl malonate is 1:1.5, and the concentration of 2-benzylidene indene-1,3-dione is 0.2mol / L; the molar weight of tetrabutyl ammonium iodide is 20% of 2-benzylidene indene-1,3-dione; the molar weight concentration of tetrabutyl ammonium iodide is 0.04mol / L; 30wt% peroxide The molar amount of the hydrogen solution is three times that of 2-benzylidene indene-1,3-dione, and the ratio of the mixed solution of 1,2-dichloroethane to γ-valerolactone is 1:1.
[0079] Prepare the mixed solution of 2-benzylidene indene-1,3-dione, cyclo(ethylene)isopropyl malonate, 1,2-dichloroethane and γ-valerolactone as solution A; The mixed solution of tetrabutylammonium iodide, 30wt% hydrogen peroxide solution and 1,2-dichloroethane and γ-valerolactone is configured as solution B, and then solution A and solution B are according to the flow volume ratio of 1:1 pumped into the microchannel reaction device, the flow rate ...
Embodiment 2
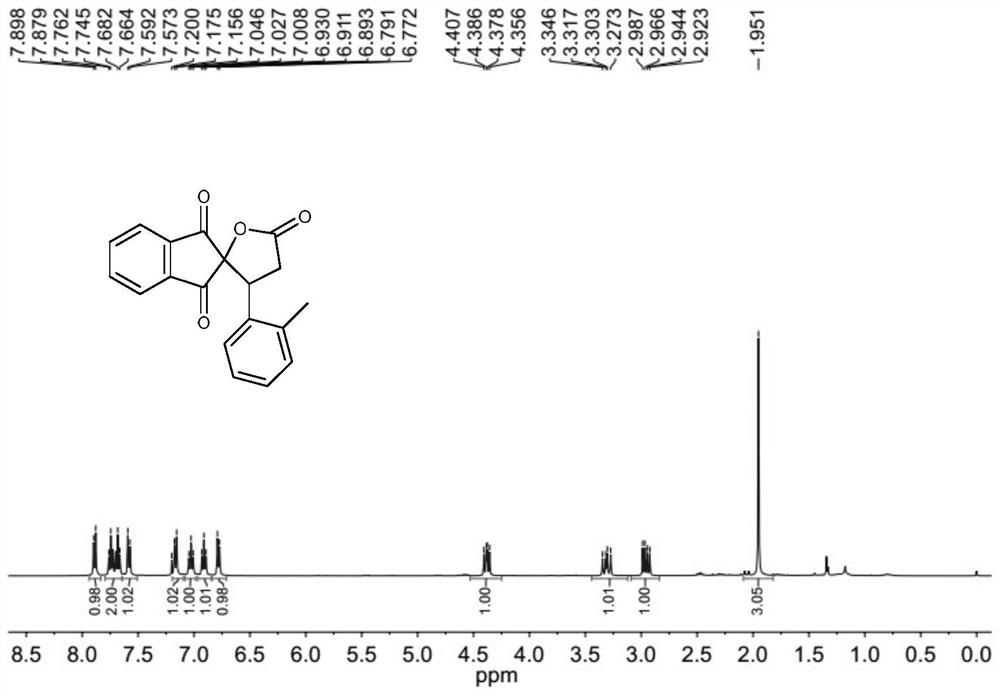
[0097] The molar ratio of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione to cyclo(methylene)isopropyl malonate is 1:1.5, 2- The concentration of [(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione is 0.2mol / L; the molar weight of tetrabutylammonium iodide is 2-[(2 20% of -methylphenyl)methylene]-1H-indene-1,3(2H)-dione; the molar concentration of tetrabutylammonium iodide is 0.04mol / L; 30wt% aqueous hydrogen peroxide The molar amount of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione is three times that of 2-[(2-methylphenyl)methylene]-1H-indene-1,3(2H)-dione, and 1,2-dichloroethane and γ-pentane The mixed solution ratio of ester is 1:1.
[0098] 2-[(2-Methylphenyl)methylene]-1H-indene-1,3(2H)-dione, cyclo(methylene)isopropyl malonate and 1,2-dichloroethane The mixed solution prepared with γ-valerolactone is designated as solution A; the mixed solution obtained by preparing tetrabutylammonium iodide, 30 wt% aqueous hydrogen peroxide and 1,2-dichloroethane and γ-...
Embodiment 3
[0100] The molar ratio of 2-[(2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione to cyclo(ethylene)isopropyl malonate is 1:1.5, 2-[ The concentration of (2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione is 0.2mol / L; the molar amount of tetrabutylammonium iodide is 2-[(2-bromo 20% of phenyl)methylene]-1H-indene-1,3(2H)-dione; the molar concentration of tetrabutylammonium iodide is 0.04mol / L; the molar quantity of 30wt% hydrogen peroxide aqueous solution 3 times that of 2-[(2-bromophenyl)methylene]-1H-indene-1,3(2H)-dione, a mixed solution of 1,2-dichloroethane and γ-valerolactone The ratio is 1:1.
[0101] 2-[(2-Bromophenyl)methylene]-1H-indene-1,3(2H)-dione, cyclo(methylene)isopropylmalonate and 1,2-dichloroethane were mixed with The mixed solution configuration of γ-valerolactone is denoted as solution A; Be solution B, then solution A and solution B are pumped in the microchannel reaction device according to the flow volume ratio of 1:1, and the flow rate is 2.5mL / min, ente...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com