Benzothiazole derivative and synthesis method and application thereof
A technology of benzothiazole and synthesis method, which is applied in the field of benzothiazole derivatives, can solve problems such as failure to achieve subcellular organelle localization, and achieve the effects of high sensitivity, good selectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
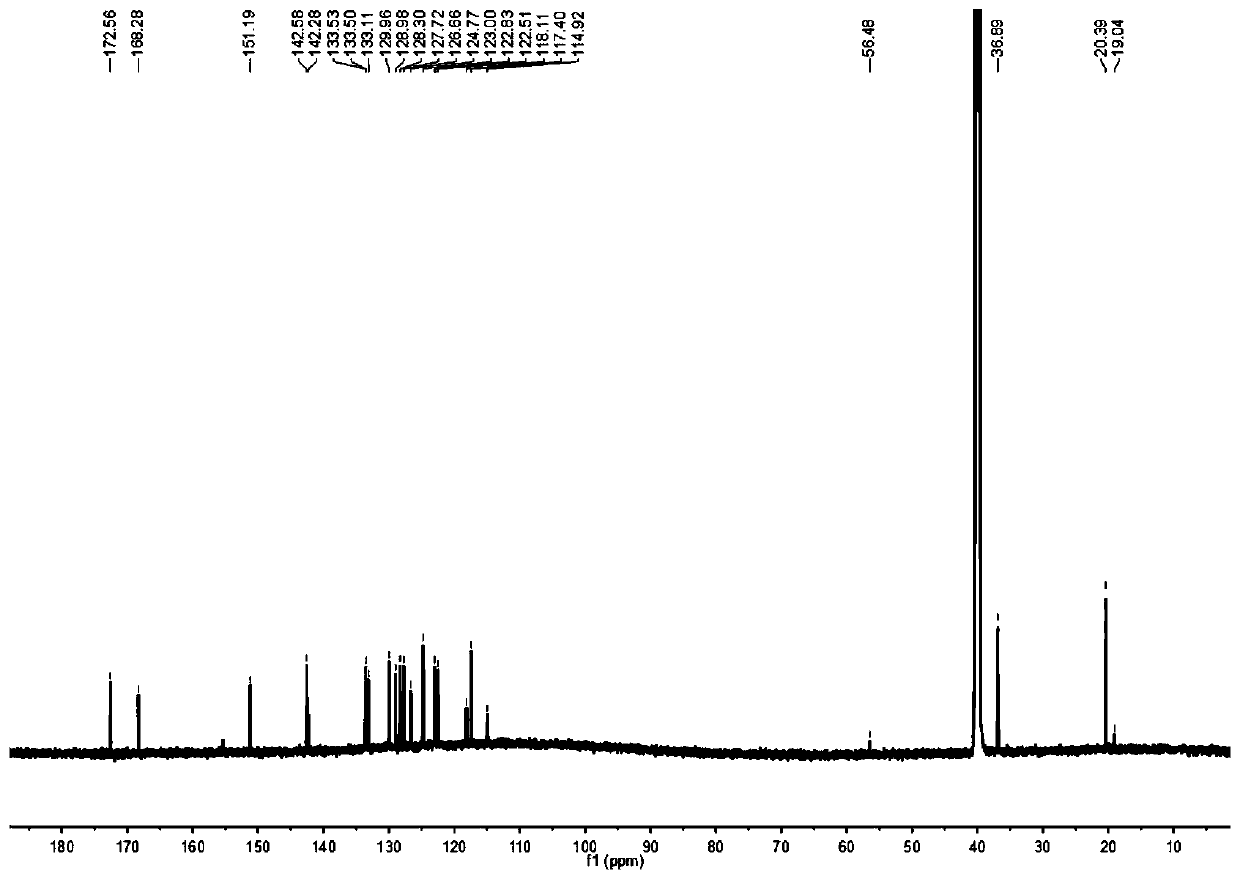
[0038] Preparation and characterization of embodiment 1NSSN
[0039] 2-Aminothiophenol (0.625g, 5mmol) and 5-methyl salicylaldehyde (0.681g, 5mmol) were mixed in 15mL dimethyl sulfoxide, silver nitrate (0.009g, 0.05mmol) was added, and the mixture After reacting at room temperature for 2 hours, dilute with dichloromethane, wash with brine, combine the organic phases, remove the solvent under reduced pressure, use petroleum ether and ethyl acetate at a volume ratio of 6:1 as the eluent, separate by silica gel column chromatography, and purify to obtain Pale yellow powdery solid (0.893g, yield: 74.07%). 1 H NMR (600MHz, Chloroform-d) δ8.01(d, J=8.1Hz, 1H), 7.93(d, J=7.9Hz, 1H), 7.56-7.48(m, 2H), 7.43(t, J= 7.5Hz, 1H), 7.21(d, J=8.3Hz, 1H), 7.04(d, J=8.4Hz, 1H), 2.37(s, 3H). 13 C NMR (150MHz, Chloroform-d) δ169.41, 155.79, 151.84, 133.79, 132.60, 128.72, 128.34, 126.67, 125.47, 122.12, 121.52, 117.68, 116.33, 20.51.
[0040]2-(Benzo[d]thiazol-2-yl)-4-methylphenol (0.455g, 1.8m...
Embodiment 2
[0044] Prepare a fluorescent probe stock solution of 2mM NSSN with dimethylsulfoxide (DMSO); mix 2mL of PBS / CH 3 Add CN (1:1, v / v, pH=7.4) solution and 10 μL fluorescent probe stock solution into a fluorescent cuvette, take hypochlorous acid solution, and gradually add it to the cuvette with a micro-sampler, While adding the sample, it was detected on the fluorescence spectrophotometer. With the addition of hypochlorous acid (0-30μM), the fluorescence intensity at 670nm gradually weakened, and the fluorescence intensity at 540nm gradually increased ( Figure 4a ), the fluorescence showed a ratiometric change. When hypochlorous acid (30-120μM) was added, the fluorescence intensity gradually increased from 540nm to 552nm and stabilized ( Figure 4b ), should be a "turn-on" type change.
Embodiment 3
[0046] Prepare 2mM NSSN fluorescent probe stock solution with dimethyl sulfoxide (DMSO); add 2mL of PBS / CH to the fluorescent cuvette 3 CN (1:1, v / v, pH=7.4) solution and 10 μL fluorescent probe stock solution, and then add 10 times the equivalent of other analytes and hypochlorous acid: NSSN, Cl - ,Br - , I - ,CH 3 COO - ,NO 2 - ,NO 3 - ,Cys,Hcy,GSH,S 2 o 3 2- , SO 4 2- , SO 3 2- , HSO 3 - ,KMnO 4 , detected on a fluorescence spectrophotometer ( Figure 5 ). Hypochlorous acid makes the fluorescence intensity of the detection system increase significantly at 552nm, and other analytes basically do not cause changes in the fluorescence intensity of the detection system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com