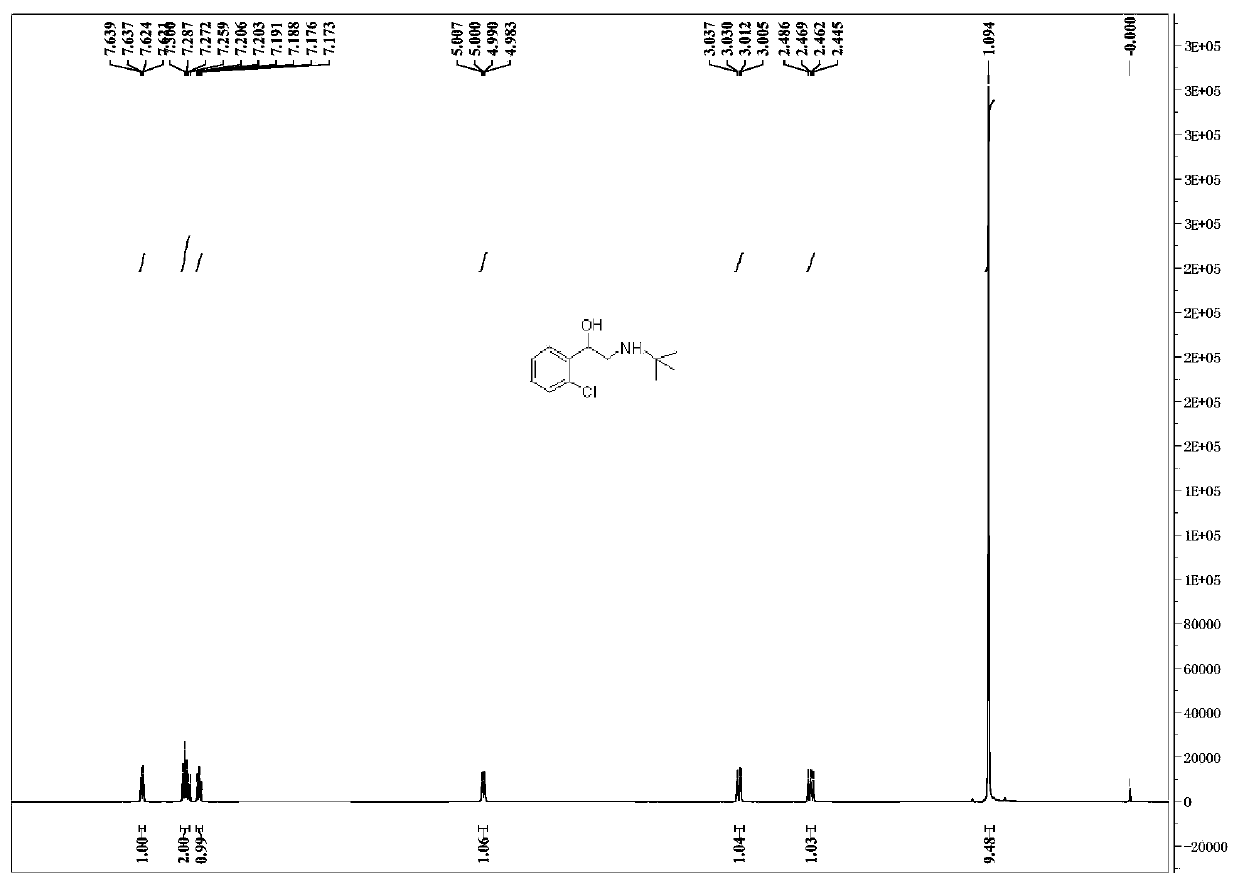
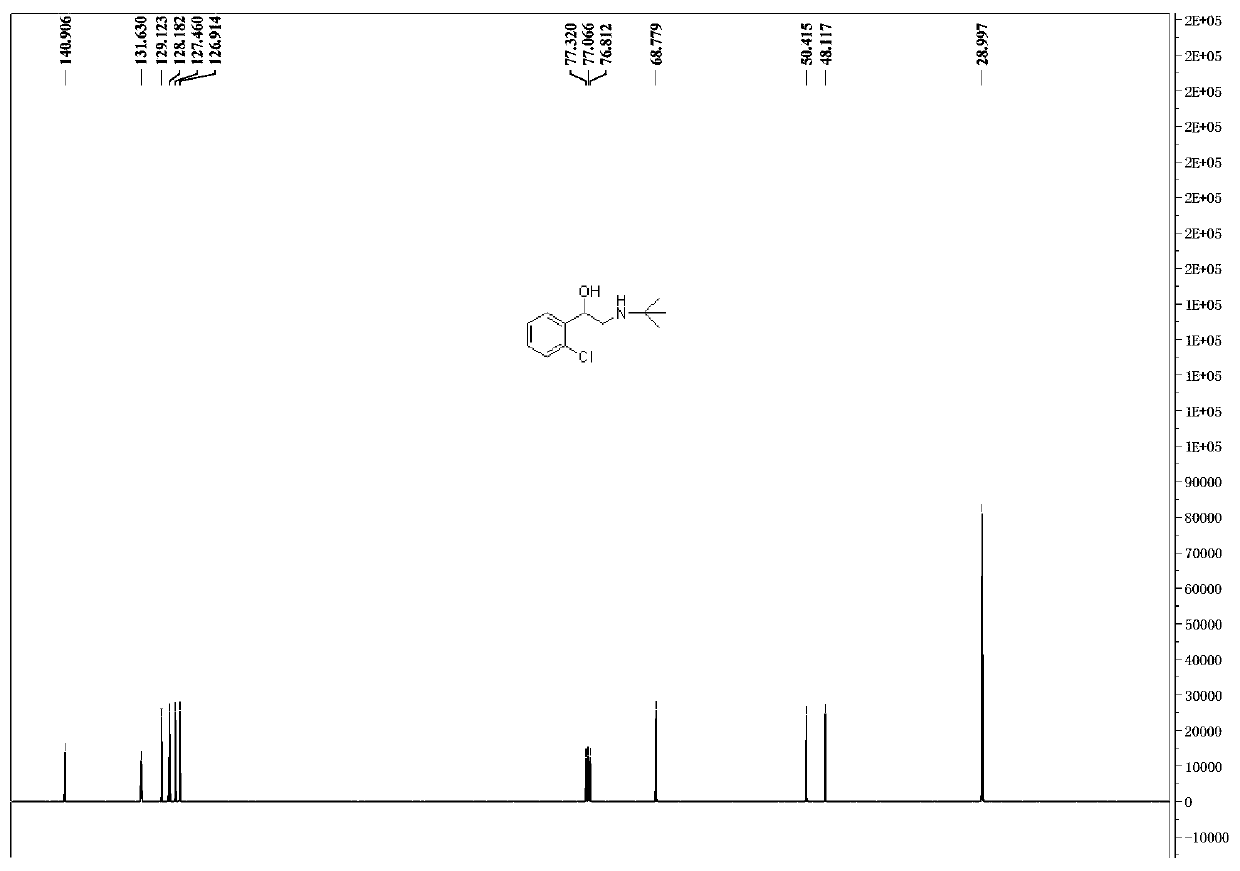
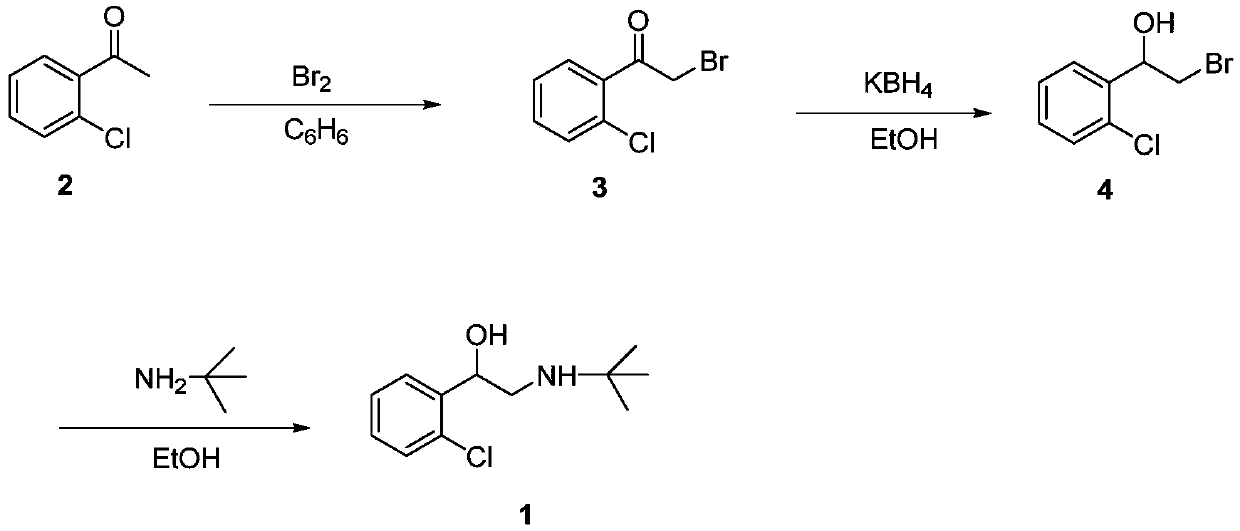
Preparation method of tulobuterol
A technology of tulobuterol and chlorophenyl, which is applied in the field of preparing and synthesizing tulobuterol, can solve problems such as potential safety hazards and cumbersome post-processing, and achieve the effects of improving production safety, simplifying process operation, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the synthesis of 1-(2-chlorophenyl)-2-bromoethanol
[0043] In a 500mL round-bottomed flask, dissolve 2-chlorostyrene (13.86g, 100mmol) in a mixed solvent of 200mL acetone and 40mL water, and slowly add dibromohydantoin (15.73g, 55mmol) under stirring at room temperature. The reaction was stirred for 2h. TLC (V 乙酸乙酯 :V 石油醚 =1:10) to detect that the reaction was complete, and after most of the acetone was evaporated under reduced pressure, extracted with dichloromethane, washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1-(2-chlorophenyl)-2 - Bromoethanol (21.43 g, 91%).
Embodiment 2
[0044] Embodiment 2: Synthesis of 1-(2-chlorophenyl)-2-bromoethanol
[0045] Dissolve 2-chlorostyrene (13.86g, 100mmol) in a mixed solvent of 200mL acetone and 100mL water in a 500mL round bottom flask, slowly add dibromohydantoin (15.73g, 55mmol) under stirring at room temperature, after the addition is complete, continue at room temperature The reaction was stirred for 4h. TLC (V 乙酸乙酯 :V 石油醚 =1:10) to detect that the reaction was complete, and after most of the acetone was evaporated under reduced pressure, extracted with dichloromethane, washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1-(2-chlorophenyl)-2 - Bromoethanol (19.78 g, 84%).
Embodiment 3
[0046] Embodiment 3: Synthesis of 1-(2-chlorophenyl)-2-bromoethanol
[0047] In a 500mL round-bottomed flask, dissolve 2-chlorostyrene (13.86g, 100mmol) in a mixed solvent of 200mL acetonitrile and 40mL water, and slowly add dibromohydantoin (15.73g, 55mmol) under stirring at room temperature. The reaction was stirred for 2h. TLC (V 乙酸乙酯 :V 石油醚 =1:10) to detect that the reaction was complete, after most of the acetonitrile was distilled off under reduced pressure, extracted with dichloromethane, washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1-(2-chlorophenyl)-2 - Bromoethanol (21.20 g, 90%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com