Synthetic method for increasing yield of 5-chloro-1-indanone
A synthesis method and technology of indanone, which is applied in the field of synthesis to improve the yield of 5-chloro-1-indanone, can solve the problems of poor selectivity, many side reactions, and low yield, and achieve improved viscosity, increased selectivity, Reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
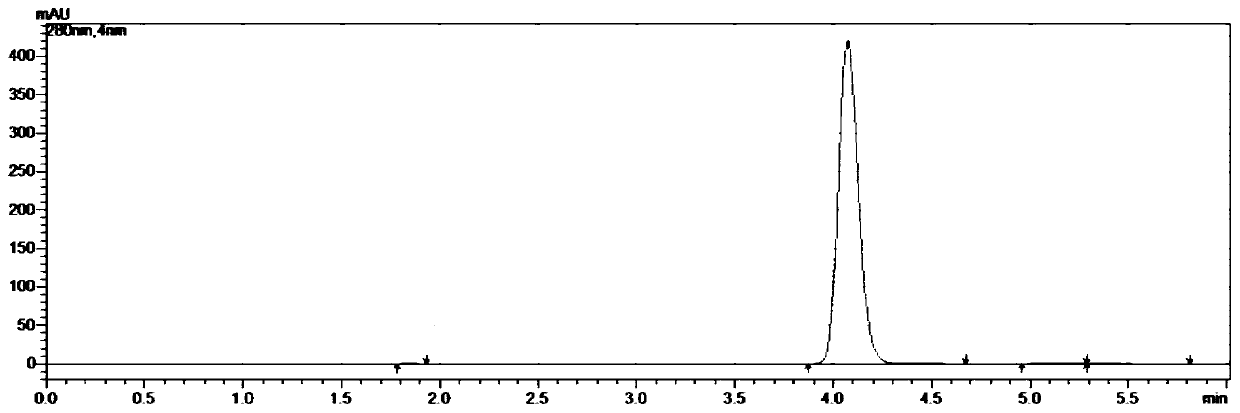
Embodiment 1
[0049] A kind of synthetic method of 5-chloro-1-indanone, concrete steps are as follows:
[0050] (1) Weigh 211.0g of 3,4'-dichloropropiophenone and 400.5g of aluminum trichloride into the reaction flask, connect the tail gas absorption device, start stirring, and slowly raise the temperature to 100°C, the system is in a molten state;
[0051] (2) When the system is in a molten state, take 21.1g tetrabutylammonium bromide, add it to the reaction flask, continue to heat up, and heat up to 160°C in 1h; after reaching the target temperature, keep it warm for 2h, and monitor the reaction with HPLC. When there is no 3,4'-dichloropropiophenone remaining, stop the reaction;
[0052] (3) Cool the reaction solution to 80°C, transfer it to water at 0-5°C, and stir for 1 hour;
[0053] (4) Suction filtration, the filter cake was rinsed with water until the pH of the filtrate=6~7, and suction filtration until no water drops fell to obtain 337.2g of crude product;
[0054](5) Place the c...
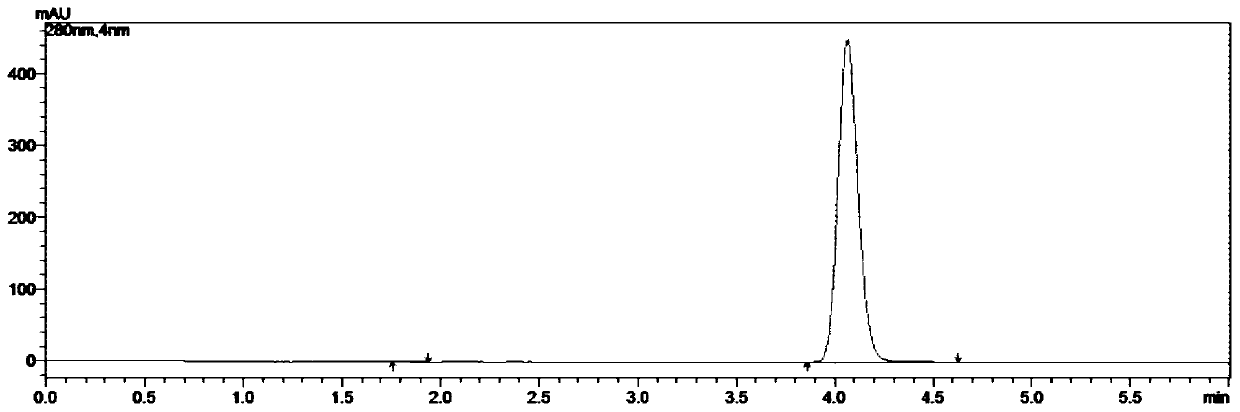
Embodiment 2
[0063] A kind of synthetic method of 5-chloro-1-indanone, concrete steps are as follows:
[0064] (1) Weigh 211.0g of 3,4'-dichloropropiophenone and 400.5g of aluminum trichloride into the reaction flask, connect the tail gas absorption device, start stirring, and slowly raise the temperature to 100°C, the system is in a molten state;
[0065] (2) When the system is in a molten state, take 1.06g of tetrabutylammonium bromide, add it to the reaction flask, continue to heat up, and heat up to 160°C in 1h; after reaching the target temperature, keep it warm for 4.5h, and monitor the reaction with HPLC. At this time, there is no 3,4'-dichloropropiophenone remaining, and the reaction is stopped;
[0066] (3) Cool the reaction solution to 80°C, transfer it to water at 0-5°C, and stir for 1 hour;
[0067] (4) Suction filtration, the filter cake was rinsed with water until the pH of the filtrate=6~7, and suction filtration until no water drops fell to obtain 311.8g of crude product; ...
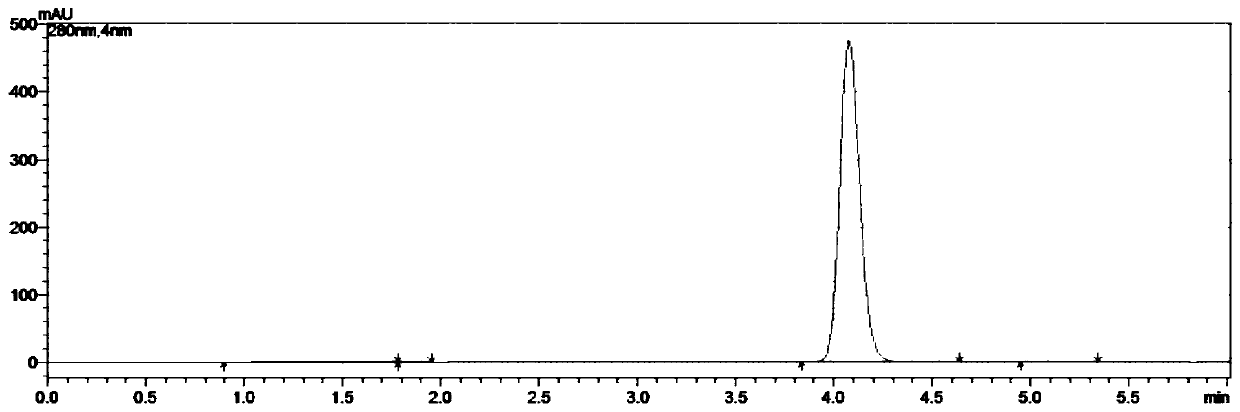
Embodiment 3
[0073] A kind of synthetic method of 5-chloro-1-indanone, concrete steps are as follows:
[0074] (1) Weigh 211.0g of 3,4'-dichloropropiophenone and 400.5g of aluminum trichloride into the reaction flask, connect the tail gas absorption device, start stirring, and slowly raise the temperature to 100°C, the system is in a molten state;
[0075] (2) When the system is in a molten state, take 21.1g of tetrabutylammonium chloride, add it to the reaction flask, continue to heat up, and heat up to 160°C in 1h; after reaching the target temperature, keep it warm for 4h, and monitor the reaction with HPLC. When there is no 3,4'-dichloropropiophenone remaining, stop the reaction;
[0076] (3) Cool the reaction solution to 80°C, transfer it to water at 0-5°C, and stir for 1 hour;
[0077] (4) Suction filtration, the filter cake was rinsed with water until the pH of the filtrate=6~7, and suction filtration until no water drops fell to obtain 317.6g of crude product;
[0078] (5) Place ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com