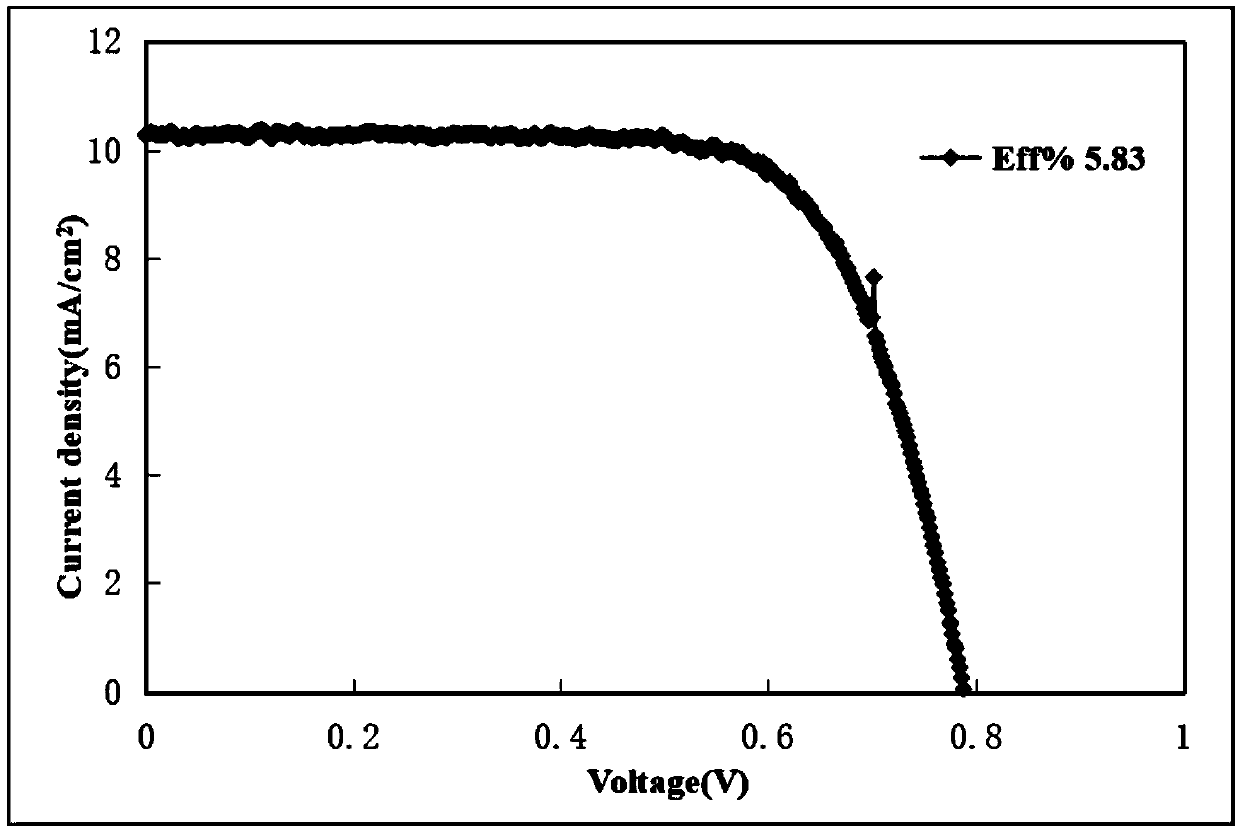
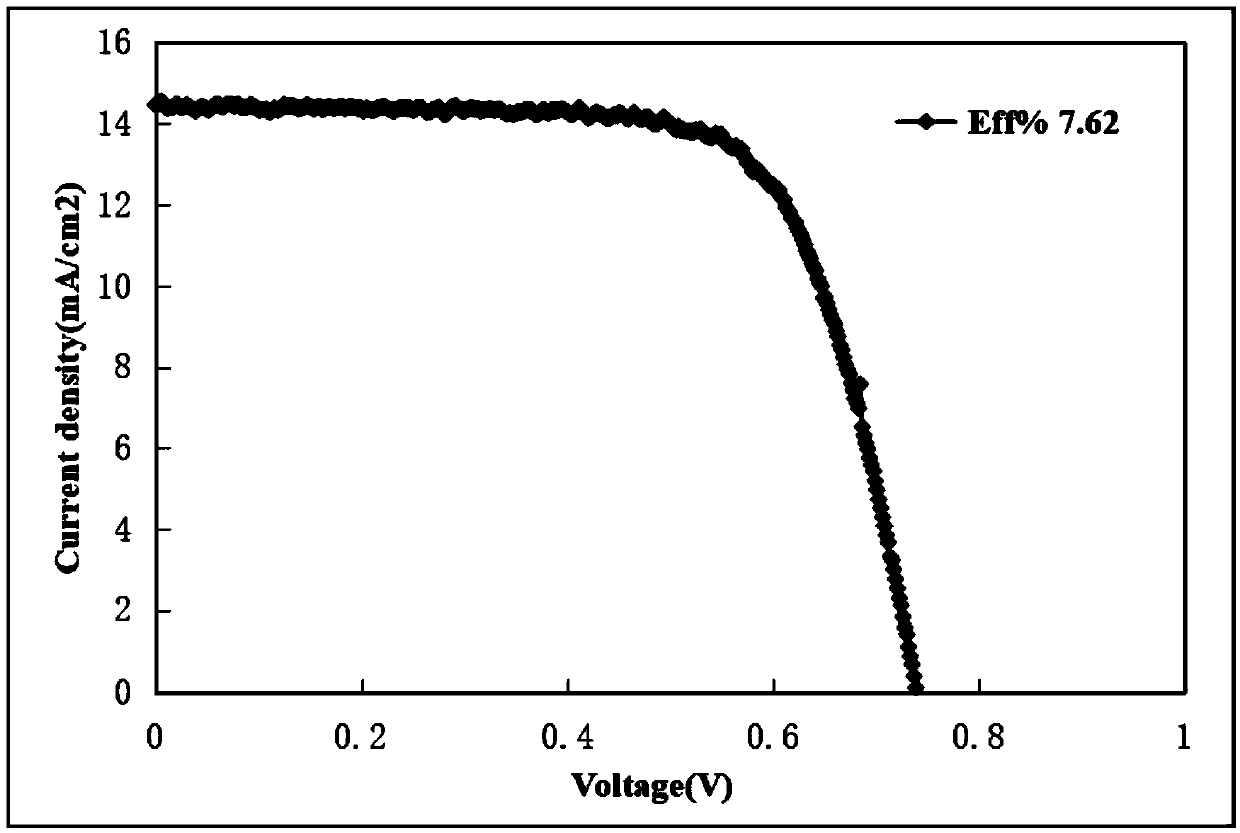
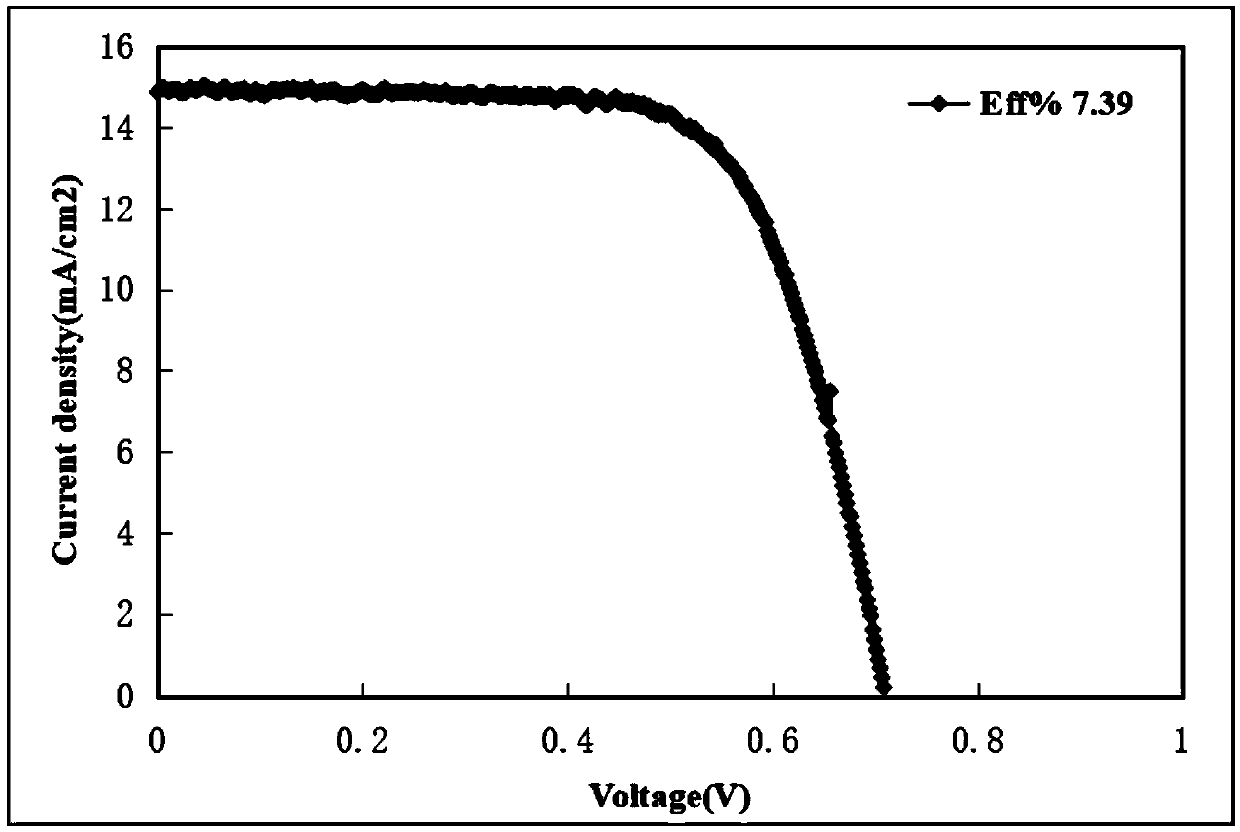
D-pi-A type small organic molecular dye based on di (4-styrylphenyl) aniline, synthetic method and application thereof
A technology of styrylphenyl and small molecules, which is applied in the preparation of organic dyes, organic compounds, organic chemistry, etc., can solve the problems of less than 1% total yield, high synthesis time cost and economic cost, and achieve low synthesis cost , expanding the conjugation range, and excellent photoelectric performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Concrete, the synthetic method of the D-π-A type organic small molecule dye based on two (4-styrylphenyl) anilines in the present invention, its synthetic steps are as follows:
[0034] (1) Under nitrogen protection conditions, dissolve 4-bromotriphenylamine in N,,N'-dimethylformamide (DMF), and control the reaction temperature below 0°C; after a period of reaction, add to the above reaction system Phosphorus oxychloride was added to the mixture, and then the reaction temperature was controlled at 80-100°C, and the reaction time was 12-24 hours; the reaction was quenched by adding water in an ice-water bath, and after suction filtration, extraction, and solvent removal, the residue was separated and purified with a silica gel column , to obtain intermediate compound a.
[0035] (2) Under the condition of nitrogen protection, compound a and diethyl benzyl phosphate were formulated into a DMF solution, stirred for 30 minutes to dissolve it, and potassium tert-butoxide was...
Embodiment 1
[0038] Embodiment 1: the preparation of dye molecular intermediate b
[0039] The preparation method of the D-π-A type organic small molecule dye intermediate b based on two (4-styrylphenyl) anilines in the present invention, its synthetic steps are as follows:
[0040] Under nitrogen protection, add 4-bromotriphenylamine (324mg, 1.0mmol) and N,N'-dimethylformamide (DMF, 2mL) into the dry reactor, control the temperature below 0°C and stir and mix for 15min , followed by dropwise addition of phosphorus oxychloride (POCl 3 , 2.33mL, 25mmol), the mixture was stirred at 100°C for 12h. Stop the reaction and cool in an ice-water bath, slowly add water to dilute and quench, filter under reduced pressure to take the filter cake, extract with dichloromethane / water system, collect the organic phase and dry with anhydrous sodium sulfate, filter and remove the solvent under reduced pressure. The residue was separated and purified by silica gel column (eluent: petroleum ether / dichlorome...
Embodiment 2
[0042] Example 2: Synthesis and preparation of E-1 dye molecule with benzene ring as conjugated π bridge
[0043] A kind of D-π-A type organic small molecule dye precursor compound d1 and its corresponding dye E-1 based on two (4-styrylphenyl) aniline in the present invention, its molecular structure is as follows:
[0044]
[0045] The synthesis of the E-1 dye molecule and its precursor d1 in this embodiment comprises the following steps:
[0046] Under nitrogen protection conditions, compound b (350mg, 0.66mmol), 4-formylphenylboronic acid (119mg, 0.79mmol), tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 , 38mg, 0.033mmol), saturated potassium carbonate solution (0.8mL) and tetrahydrofuran (5.5mL) were sequentially added into the reactor, the reaction temperature was controlled at 75°C, and the reaction was stirred and refluxed for 12h. After stopping the reaction, the reaction mixture was cooled to room temperature, filtered under reduced pressure to get the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com