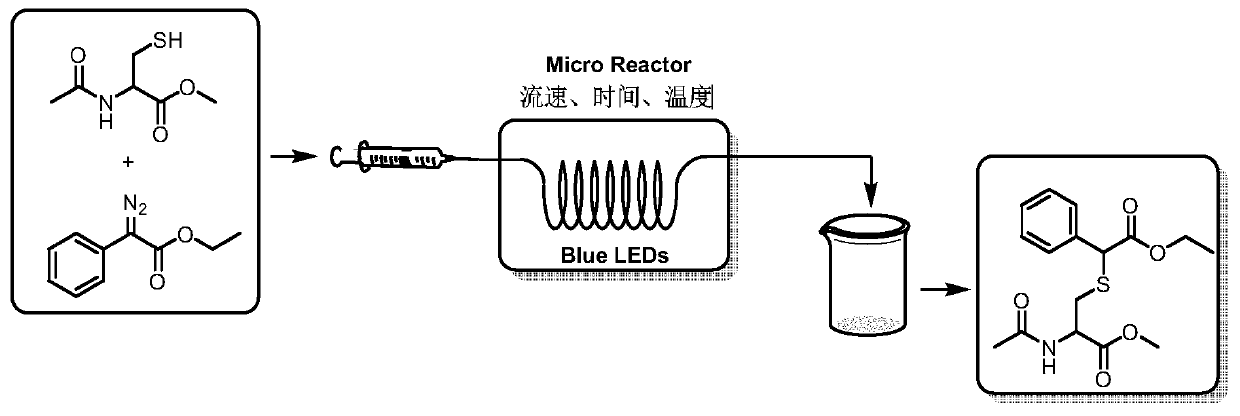
Method for preparing amino acid derivative by utilizing photocatalysis micro-channel
A technology of derivatives and amino acids, applied in the field of chemical synthesis, can solve the problems of unfriendly environment, many synthesis steps, and limited application, and achieve the effects of shortened reaction time, simple operation, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
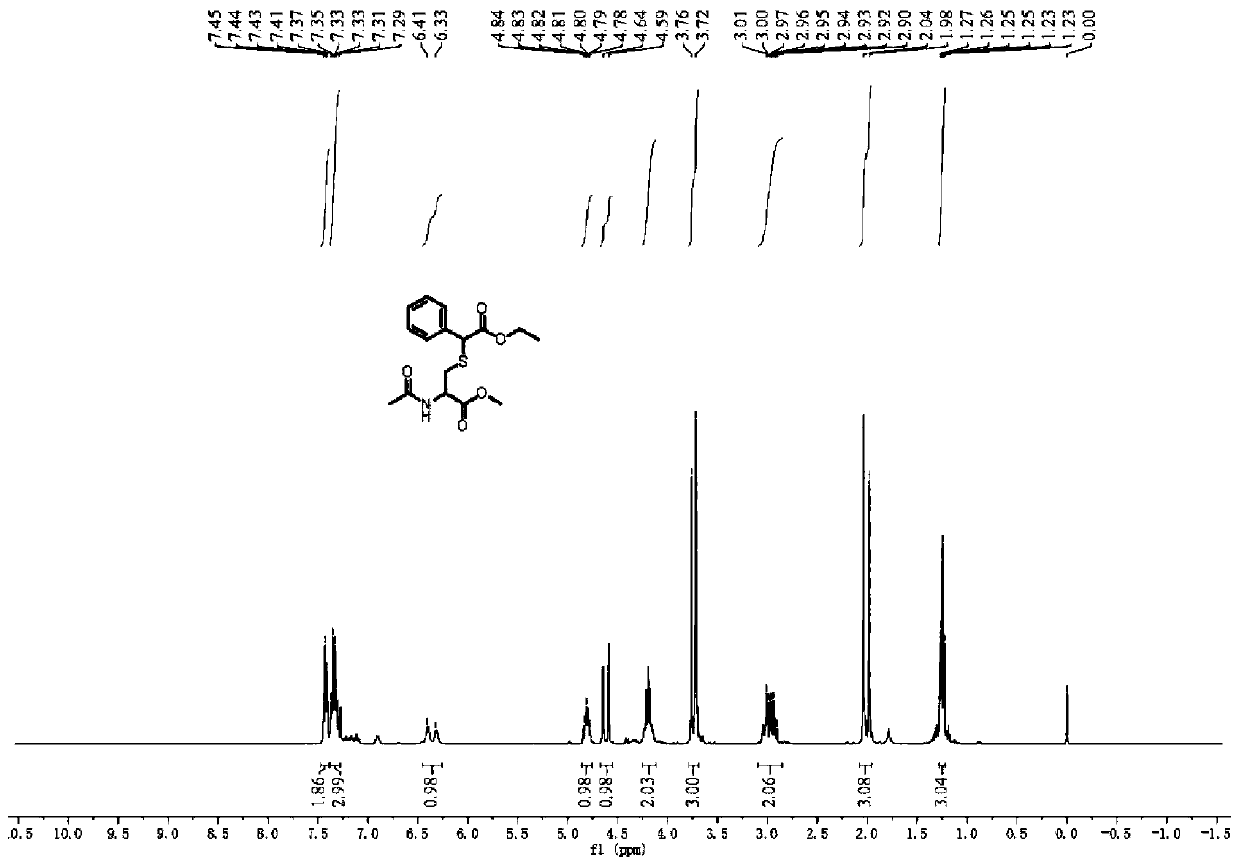
[0051] Weigh 0.0885g (0.5mmol, 1.0equiv) of N-acetyl-L-cysteine methyl ester, 0.190g (1.0mmol, 2.0equiv) of ethyl 2-diazo-2-phenylacetate, and use 2.5mL di The methyl chloride is dissolved and loaded into the syringe after complete dissolution. The reaction solution is pumped into a reactor with a coil inner diameter of 0.5mm, the volume is 1mL, the flow rate of the microreactor is 0.1mL / min, irradiated with a blue LED light source (50W, 455nm), and the reaction is carried out at 25°C. The residence time 10min. After the reaction, TLC detection was performed, and 156.0 mg of the final product was obtained by column chromatography (petroleum ether: ethyl acetate = 1:1), with a yield of 92%. Such as image 3 , Figure 4 As shown, the characterization data are as follows: 1 H NMR (400MHz, Chloroform-d) δ7.48–7.39(m,2H),7.38–7.28(m,3H),6.37(d,J=33.7Hz,1H),4.86–4.76(m,1H), 4.67–4.55(m,1H),4.25–4.12(m,2H),3.74(d,J=16.6Hz,3H),2.96(s,2H),2.01(d,J=23.1Hz,3H),1.28 –1...
Embodiment 2
[0053] Weigh 0.0885g (0.5mmol, 1.0equiv) of N-acetyl-L-cysteine methyl ester, 0.190g (1.0mmol, 2.0equiv) of ethyl 2-diazo-2-phenylacetate, and use 2.5mL di The methyl chloride is dissolved and loaded into the syringe after complete dissolution. The reaction solution is pumped into a reactor with a coil inner diameter of 0.5mm, the volume is 1mL, the flow rate of the microreactor is 0.1mL / min, irradiated with a blue LED light source (10W, 455nm), the reaction is carried out at 25°C, and the residence time 10min. After the reaction was completed, TLC detection was performed, and 128.9 mg of the product was obtained by column chromatography (petroleum ether: ethyl acetate = 1:1), with a yield of 76%.
Embodiment 3
[0055] Weigh 0.0885g (0.5mmol, 1.0equiv) of N-acetyl-L-cysteine methyl ester, 0.190g (1.0mmol, 2.0equiv) of ethyl 2-diazo-2-phenylacetate, and use 2.5mL di The methyl chloride is dissolved and loaded into the syringe after complete dissolution. The reaction solution is pumped into a reactor with a coil inner diameter of 0.5mm, the volume is 1mL, the flow rate of the microreactor is 0.1mL / min, irradiated with a blue LED light source (50W, 455nm), and the reaction is carried out at 15°C. The residence time 10min. After the reaction was completed, TLC detection was performed, and 140.8 mg of the product was obtained by column chromatography (petroleum ether: ethyl acetate = 1:1), with a yield of 83%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com