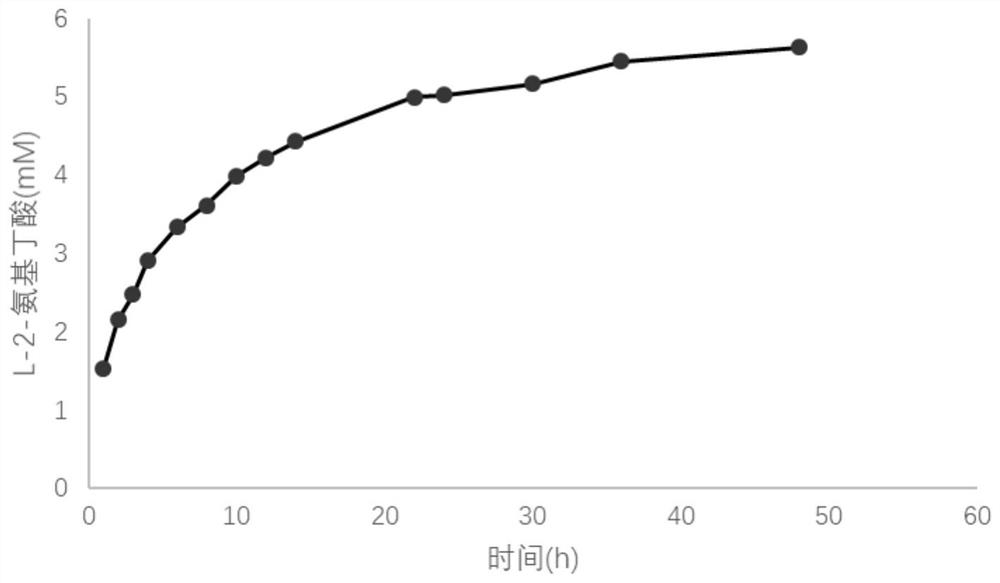
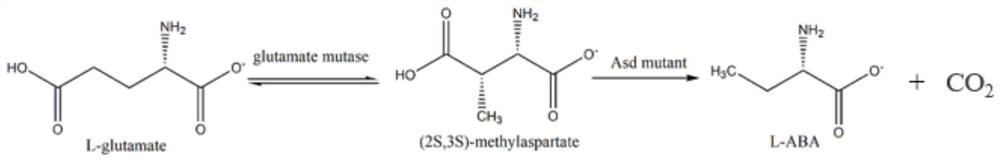
A kind of method for preparing l-2-aminobutyric acid with double enzymes in series
A kind of aminobutyric acid and double-enzyme technology, applied in the field of bioengineering, can solve the problems of unsuitability for industrial production, high substrate price of amino donor and cofactor regeneration system, and only 50% of theoretical yield, and achieve low price and low cost. The effect of simple production cost and process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Construction of recombinant Escherichia coli BL21 / pET-28-GlmES
[0024]The fusion L-glutamate mutase is connected by (glycine-glutamine) repeated decapeptides from the glmE subunit from Clostridium tetanomorphum and the mutS subunit from Clostridium cochlearium, and the nucleotide sequence is as SEQ ID NO 5, the gene was synthesized by Suzhou Jinweizhi Company and linked to pET-28a. The recombinant plasmid pET-28a-glmES was transformed into Escherichia coli BL21 strain to obtain recombinant Escherichia coli BL21 / pET-28a-glmES.
Embodiment 2
[0025] Example 2 Construction of recombinant Escherichia coli expressing L-aspartic acid-β-decarboxylase
[0026] The gene encoding the L-aspartic acid-β-decarboxylase mutant shown in the nucleotide sequence as SEQ ID NO.6 or SEQ ID NO.7 or SEQ ID NO.8 or SEQ ID NO.9 is connected to Plasmid pET-28a, obtain recombinant plasmid pET-28a-K18A / V287I or pET-28a-K18A / V287L or pET-28a-K18S / V287I or pET-28a-K18S / V287L, and transfer the recombinant plasmid into Escherichia coli BL21, Recombinant Escherichia coli BL21 / pET-28a-K18A / V287I or BL21 / pET-28a-K18A / V287L or BL21 / pET-28a-K18S / V287I or BL21 / pET-28a-K18S / V287L were obtained by screening.
Embodiment 3
[0027] Expression of embodiment 3 L-glutamic acid mutase
[0028] Recombinant Escherichia coli BL21 / pET-28a-glmES was inoculated in 5 mL of LB medium with a kanamycin concentration of 50 μg / mL, and cultured overnight at 37° C. with shaking at 200 rpm. The above overnight culture was inoculated into 2YT medium containing 50 μg / mL of kanamycin at an inoculum size (V / V) of 1%, and cultured with shaking at 37°C and 200 rpm until the bacterial liquid OD 600 To 0.6-0.8, add IPTG to a final concentration of 0.2mmol / L, induce culture at 30°C for about 20h to obtain bacteria. After collecting the bacteria by centrifugation at 6000rpm, they were ultrasonically crushed, purified by His Trap HP affinity column, and detected the target protein by SDS-PAGE.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com