Method for determining content of genotoxic impurities of ligustrazine hydrochloride
A Ligustrazine hydrochloride and genotoxicity technology, which is applied in the field of determination of Ligustrazine hydrochloride genotoxic impurities, can solve the problems of not including genotoxic impurity determination methods, unable to fully reflect the product quality status, etc., to ensure effectiveness and consistency Reliability, stable measurement conditions, comprehensive effect monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
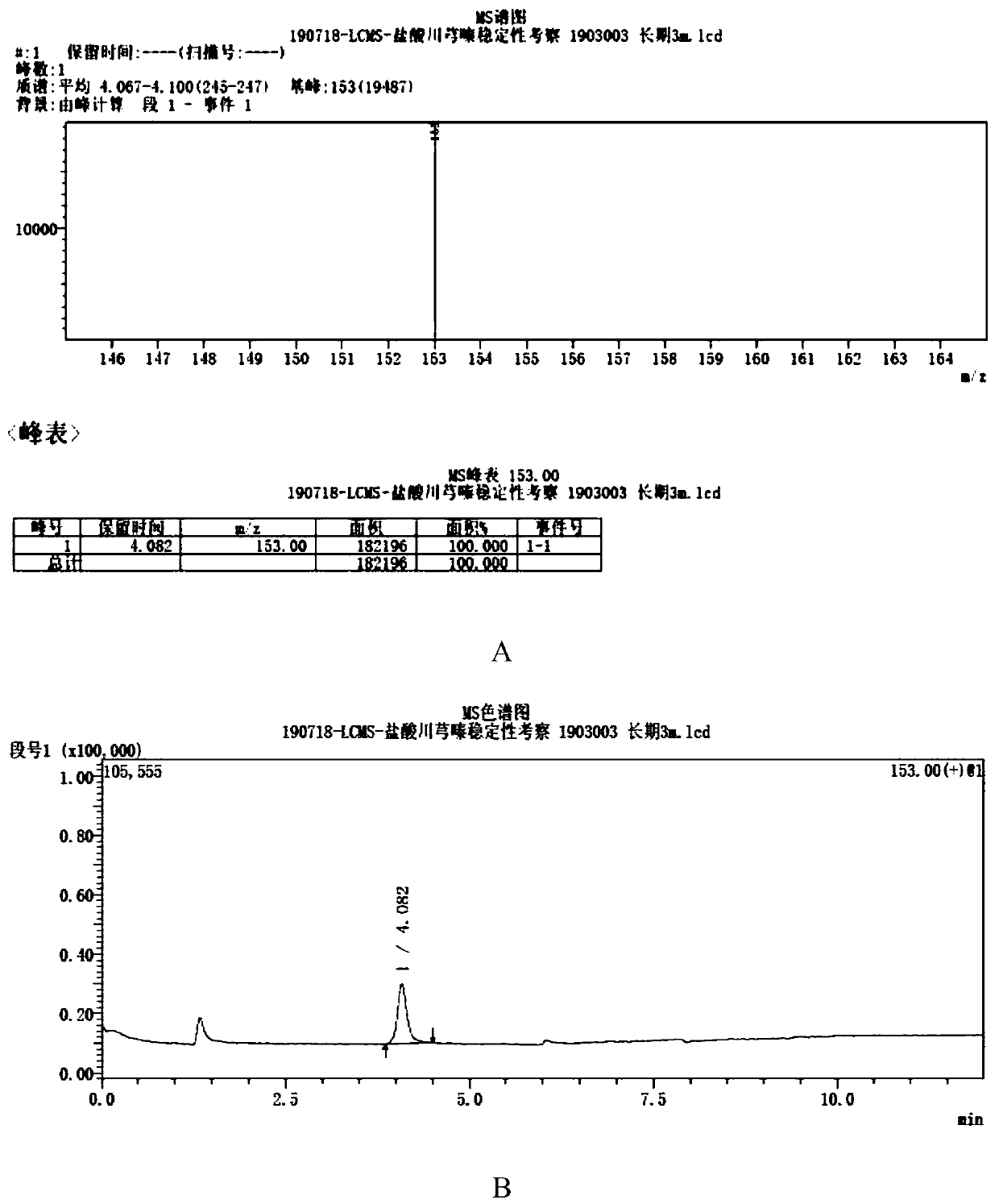
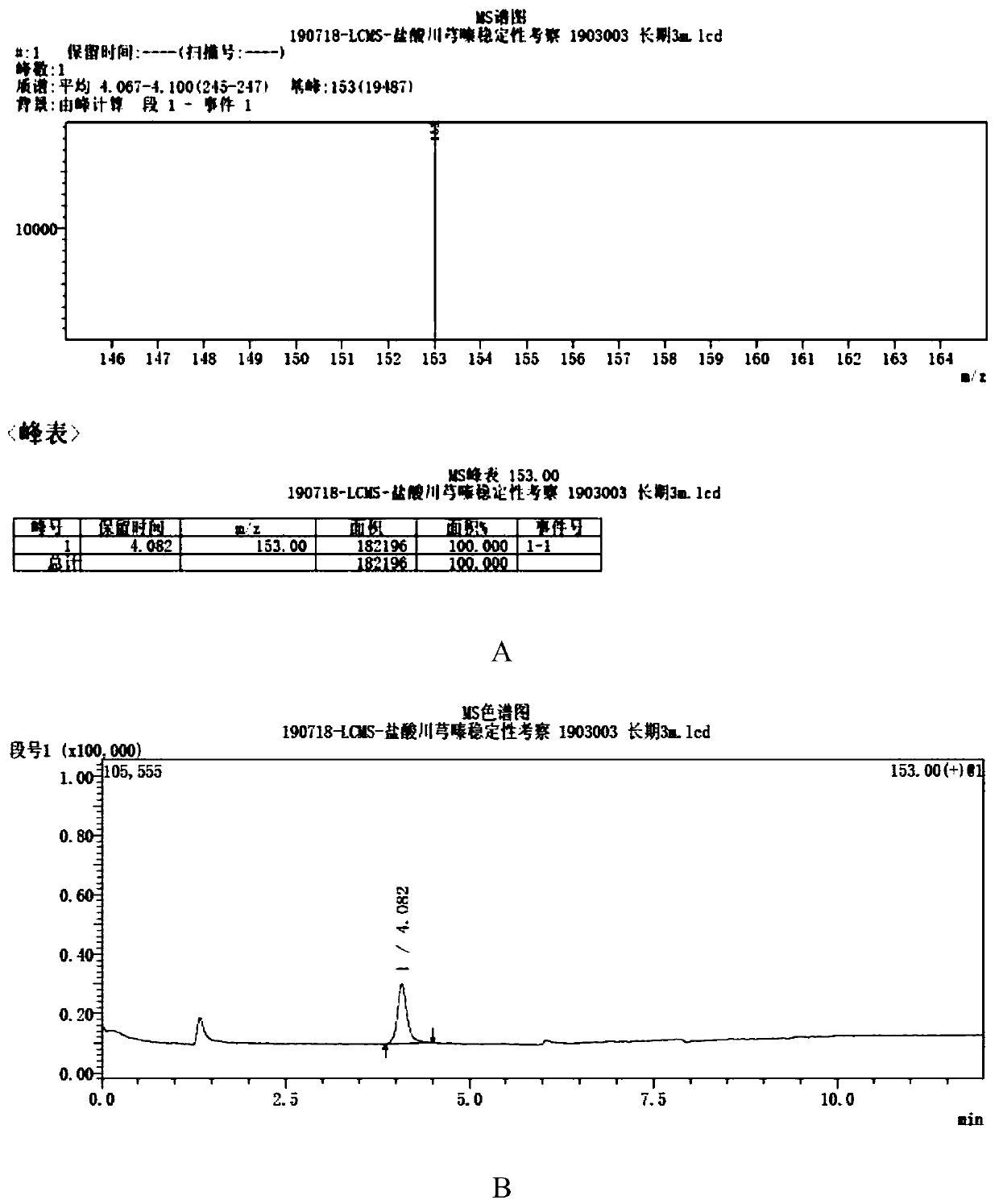
[0027] A method for measuring the genotoxic impurity content of ligustrazine hydrochloride, which adopts liquid chromatography-mass spectrometry, and its chromatographic conditions include: the chromatographic column is an octadecylsilane bonded silica gel chromatographic column, the length is 75mm, and the mobile phase is methanol and The volume ratio of water is a mixed solution of 30:70, the flow rate is 0.2ml / min; the column temperature is 40°C, the quadrupole mass spectrometer detector, the atmospheric pressure chemical ionization is used as the ion source, and the selected ion (SIM) 153m / z positive For ion mode detection, the drying gas flow rate is 10L / min, the atomizing gas flow rate is 4.0L / min, the ion source voltage is 4.5KV, and the ion source temperature is 350°C.
[0028] Sample preparation:
[0029] The test solution is: take an appropriate amount of Ligustrazine hydrochloride, accurately weighed, add mobile phase to dissolve and make a solution containing about...
Embodiment 2
[0034] A method for measuring the genotoxic impurity content of ligustrazine hydrochloride, which adopts liquid chromatography-mass spectrometry, and its chromatographic conditions include: the chromatographic column is an octadecylsilane bonded silica gel chromatographic column, the length is 75mm, and the mobile phase is methanol and The volume ratio of water is a mixed solution of 20:80, the flow rate is 0.3ml / min; the column temperature is 35°C, the quadrupole mass spectrometer detector, the atmospheric pressure chemical ionization is used as the ion source, and the selected ion (SIM) 153m / z positive For ion mode detection, the drying gas flow rate is 10L / min, the atomizing gas flow rate is 4.0L / min, the ion source voltage is 4.5KV, and the ion source temperature is 350°C.
[0035] Sample preparation is the same as in Example 1.
[0036] Test operation: Take 1 μl each of the reference substance solution and the test solution and inject it into the liquid chromatograph, and...
Embodiment 3
[0038] A method for measuring the genotoxic impurity content of ligustrazine hydrochloride, which adopts liquid chromatography-mass spectrometry, and its chromatographic conditions include: the chromatographic column is an octadecylsilane bonded silica gel chromatographic column, the length is 75mm, and the mobile phase is methanol and The volume ratio of water is a mixed solution of 25:75, the flow rate is 0.5ml / min; the column temperature is 30°C, the quadrupole mass spectrometer detector, the atmospheric pressure chemical ionization is used as the ion source, and the selected ion (SIM) 153m / z positive For ion mode detection, the drying gas flow rate is 10L / min, the atomizing gas flow rate is 4.0L / min, the ion source voltage is 4.0KV, and the ion source temperature is 360°C.
[0039] Sample preparation is the same as in Example 1.
[0040] Test operation: Take 10 μl each of the reference substance solution and the test solution and inject it into the liquid chromatograph, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com