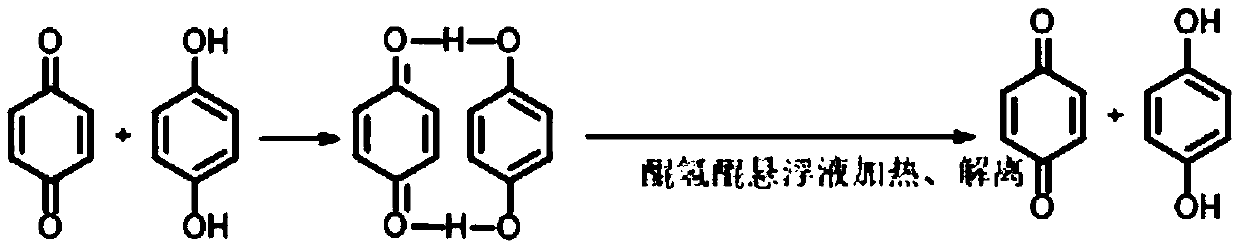
Method for preparing hydroquinone
A technology for hydroquinone and p-benzoquinone is applied in the electrochemical field of efficient preparation of hydroquinone, which can solve the problems of low concentration and high energy consumption, and achieve the effects of simple operation and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
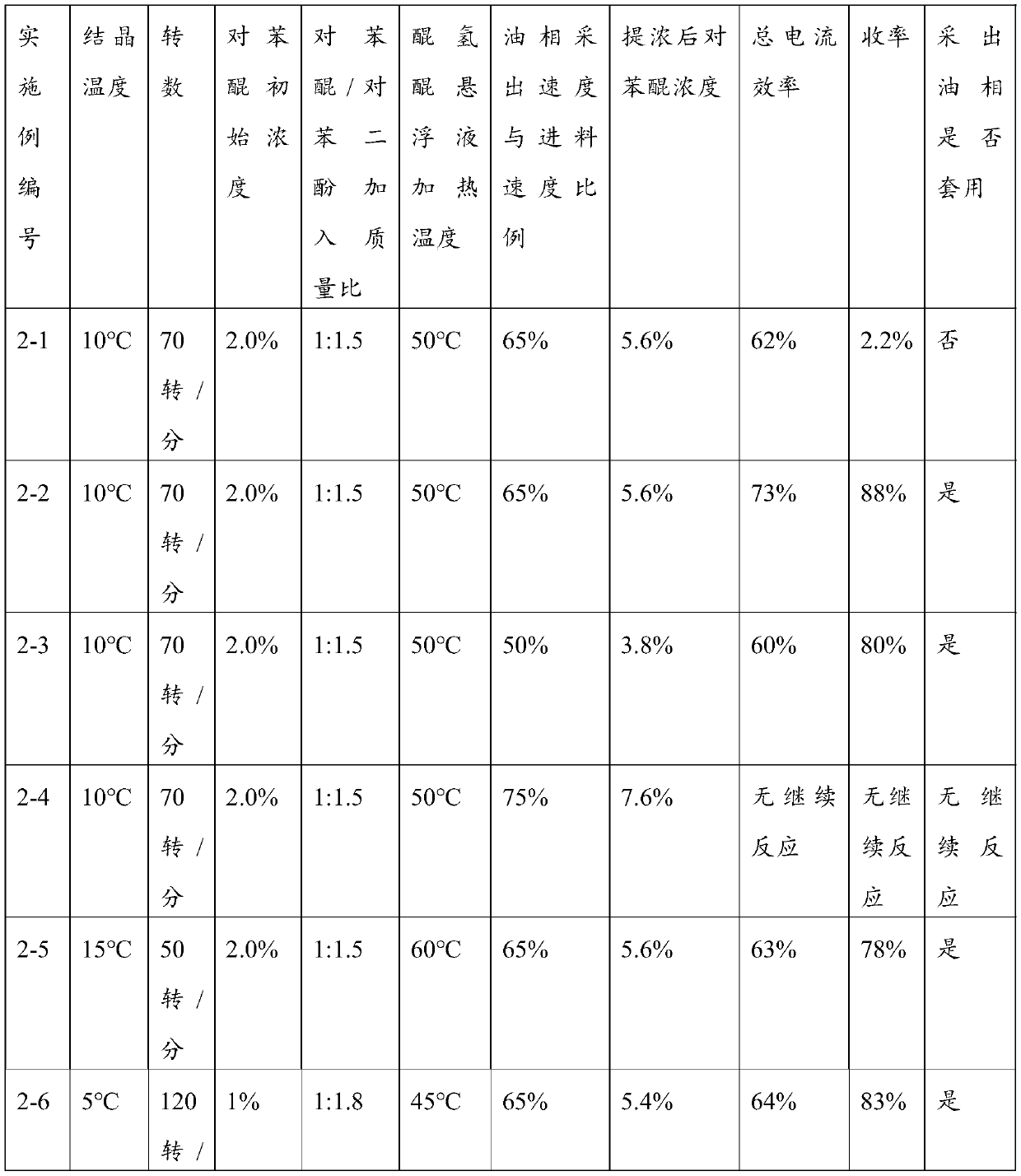
Image
Examples
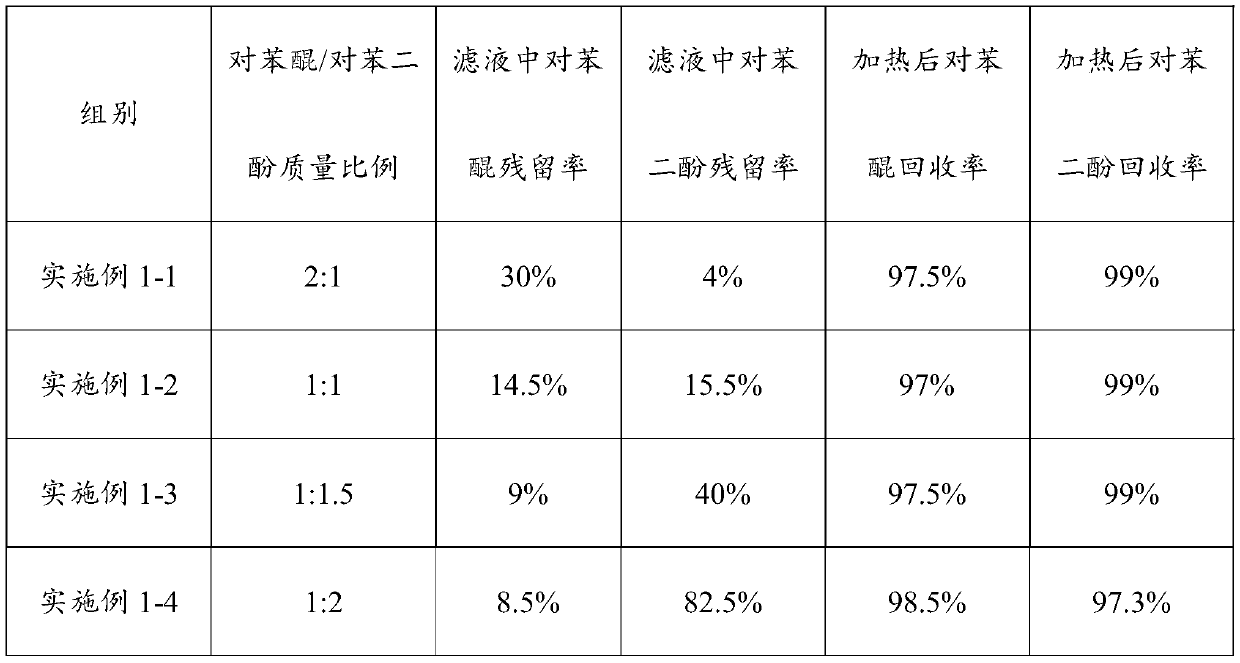
Embodiment 1-1
[0051] Take 5g of p-benzoquinone benzene solution with a p-benzoquinone concentration of 2% and 1% hydroquinone aqueous solution, wherein the mass ratio of p-benzoquinone to hydroquinone is 2:1, and both are added to a 20mL glass In the sample bottle, stir at 10°C for 15 minutes and stand still for 1 minute to obtain the reaction liquid, which includes the upper oil phase layer, the lower water phase layer and the middle layer of quinhydrone suspension, and filter the above reaction liquid to obtain the filtrate and filter cake , there are oil phase and water phase of benzene in the filtrate, use HPLC to measure the concentration of p-benzoquinone and hydroquinone in oil phase and water phase respectively, wherein the concentration of p-benzoquinone is the summation of oil phase and water phase concentration, to Hydroquinone is mainly distributed in the aqueous phase. After determination, the concentration of p-benzoquinone in the filtered filtrate was 0.6%, with a residual ra...
Embodiment 1-2
[0056] Except that the hydroquinone concentration was adjusted to 2%, wherein the mass of p-benzoquinone and hydroquinone were 1:1, other parameters were the same as those in Example 1-1.
[0057] According to the above indicators, the concentration of p-benzoquinone in the filtered solution is 0.29%, the residual rate is 14.5%, the concentration of hydroquinone is 0.31%, and the residual rate is 15.5%. The filter cake and filtrate were combined and reheated to 55° C., the concentration of p-benzoquinone was determined to be 1.94%, and the recovery rate was 97%; the concentration of hydroquinone was 1.98%, and the recovery rate was 99%.
Embodiment 1-3
[0059] Except that the hydroquinone concentration was adjusted to 3%, and the mass ratio of p-benzoquinone and hydroquinone was 1:1.5, other parameters were the same as those in Example 1-1.
[0060] The concentration of p-benzoquinone in the filtered solution was determined to be 0.18% according to the above indicators, and the residual rate was 9%. The concentration of hydroquinone is 1.2%, and the residual rate is 40%. After the filter cake and the filtrate are combined, they are reheated to 55° C., and the concentration of p-benzoquinone is 1.95%, and the recovery rate is 97.5%; the concentration of hydroquinone is 2.97%, and the recovery rate is 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com