Fluorescence labeling biological material and preparation method thereof
A biological material and fluorescent labeling technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of high biological toxicity and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
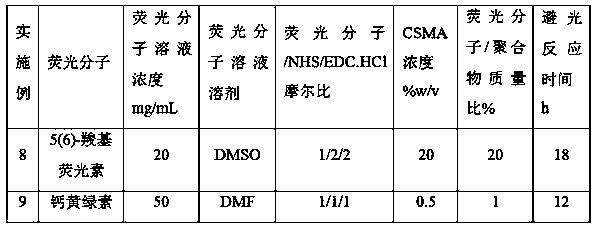
Embodiment 1
[0023] Example 1 Preparation of fluorescently labeled methacrylic anhydride modified gelatin (GelMA)
[0024] (1) Preparation of GelMA: Dissolve 10 g of gelatin in 0.01M PBS (pH=7.4) solution at 50 °C, slowly add 0.4 g of methacrylic anhydride to the solution, and at the same time adjust and maintain the pH of the solution with sodium hydroxide solution. Between 8 and 9. After the dropwise addition of methacrylic anhydride was completed, the reaction was continued for 2 hours, and then the pH of the reaction solution was adjusted to 7.4 with hydrochloric acid solution. The reaction solution was transferred into a dialysis bag with a molecular weight cut-off of 10 kDa, dialyzed in deionized water for 3 days, and then freeze-dried to obtain GelMA. Product amino substitution degree is 60%.
[0025] (2) Dissolve 1 g of rhodamine B in DMF at 10 mg / mL, then add 1.2 g of NHS and 2 g of EDC.HCl in sequence, and react at room temperature for 4 hours to obtain an activated fluorescent...
Embodiment 7
[0031] Example 7 Preparation of fluorescently labeled methacrylic anhydride modified chitosan (CSMA)
[0032] (1) Preparation of CSMA: Dissolve 10 g of chitosan in 500 mL of 2% v / v acetic acid solution. Heat to 50° C., and slowly drop 0.4 g of methacrylic anhydride into the solution. After the dropwise addition of methacrylic anhydride was completed, the reaction was continued for 2 hours, and then the pH of the reaction solution was adjusted to 7.4 with hydrochloric acid solution. The reaction solution was transferred into a dialysis bag with a molecular weight cut-off of 10 kDa, dialyzed in deionized water for 3 days, and then freeze-dried to obtain CSMA. The amino substitution degree of the product is 50%.
[0033] (2) Dissolve 1 g of Rhodamine B in DMF at 10 mg / mL, then add 1.2 g of NHS and 2 g of EDC.HCl in sequence, and react at room temperature for 4 hours to obtain an activated fluorescent molecule solution. Rhodamine B / NHS / EDC.HCl molar ratio is 1 / 1 / 1.
[0034] (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com