Rationally designed virus-like particles for modulation of chimeric antigen receptor (CAR)-t-cell therapy
A technology of parvovirus and DNA virus, applied in pharmaceutical composition, in medicine, can solve problems such as release syndrome, neurotoxicity, allergic reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0108] In the following, different aspects of the invention are defined in more detail. Each aspect so defined may be combined with one or more than one other aspect unless clearly indicated to the contrary. In particular, any feature indicated as preferred or advantageous may be combined with any other feature indicated as preferred or advantageous.
[0109] As mentioned in the background section, at worst, CAR T cell therapy can lead to TLS, leading to a so-called cytokine storm, a side effect due to the specific activity of CAR T cells. To reduce the consequences of increased cytokine release, treatment options include the use of anti-IL-6 antibodies or administration of corticosteroids, which can lead to inadequate treatment of primary diseases such as cancer. Therefore, it is generally desirable to prevent or at least reduce the likelihood of TLS during CAR T cell therapy. In the work leading up to the present invention, the inventors surprisingly discovered that AAV-de...
Embodiment 1
[0158] Example 1: Production and purification of wild-type AAV and NY-BR1-LCAR AAV
[0159] To generate the NY-BR1 AAV capsid insertion mutant used in this study, LCAR was inserted into the three-folded spike region of AAV as previously described (Michelfelder et al. (2011) PLoS ONE 6(8):e23101 .). Specifically, the oligonucleotide encoding the amino acid sequence LKNEQTLRADQMF was inserted into the VP1 position 588 in the AAV2 helper plasmid pMT-187-XX2 (the resulting nucleic acid encodes a modified VP1 having the amino acid sequence shown in SEQ ID NO: 4), Position T578 in the AAV5 helper plasmid pMT-rep2cap5-SfiI578 (the resulting nucleic acid encodes a modified VP1 with the amino acid sequence shown in SEQ ID NO: 5), position N590 in the AAV8 helper plasmid p5E18-VD2 / 8-Sfi590 (the resulting nucleic acid Encoding modified VP1 having the amino acid sequence shown in SEQ ID NO: 6) and position A589 in the AAV9 helper plasmid p5E18-VD2 / 9-Sfi589 (the resulting nucleic acid enc...
Embodiment 2
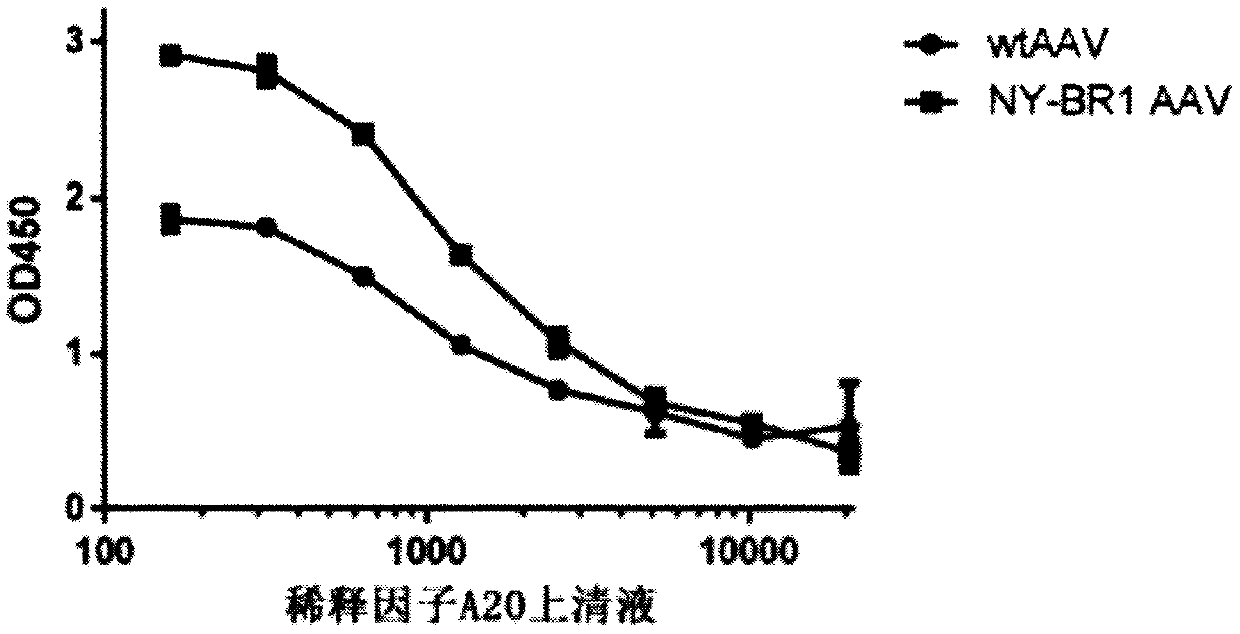
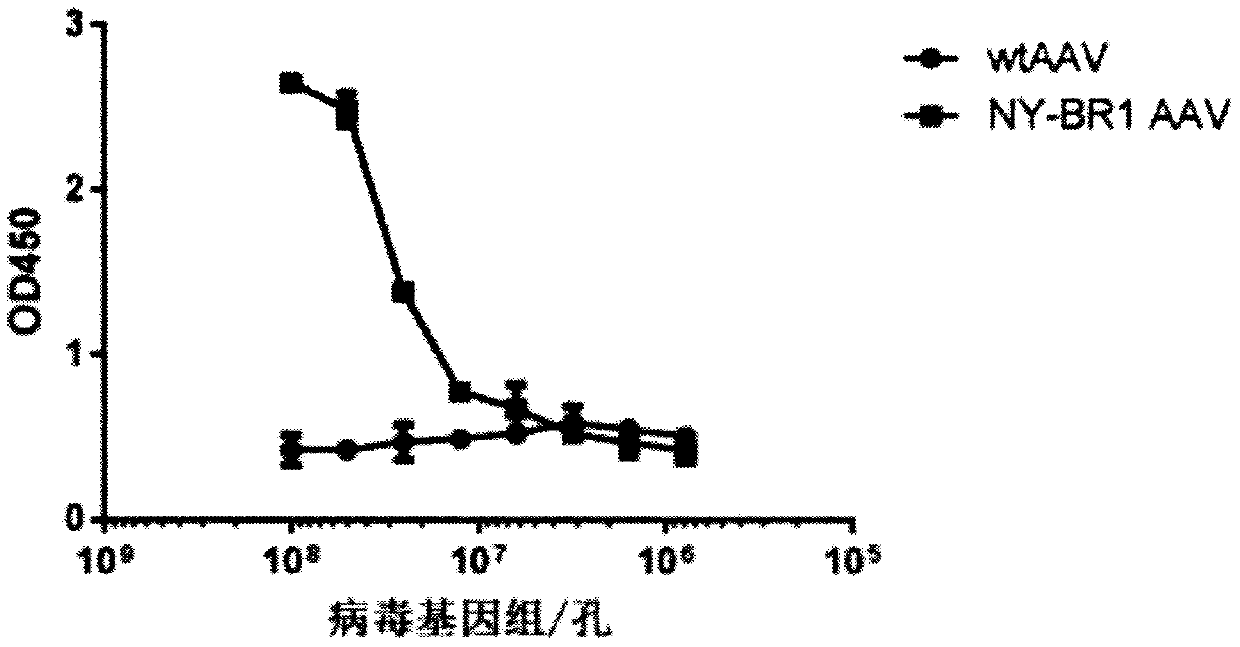
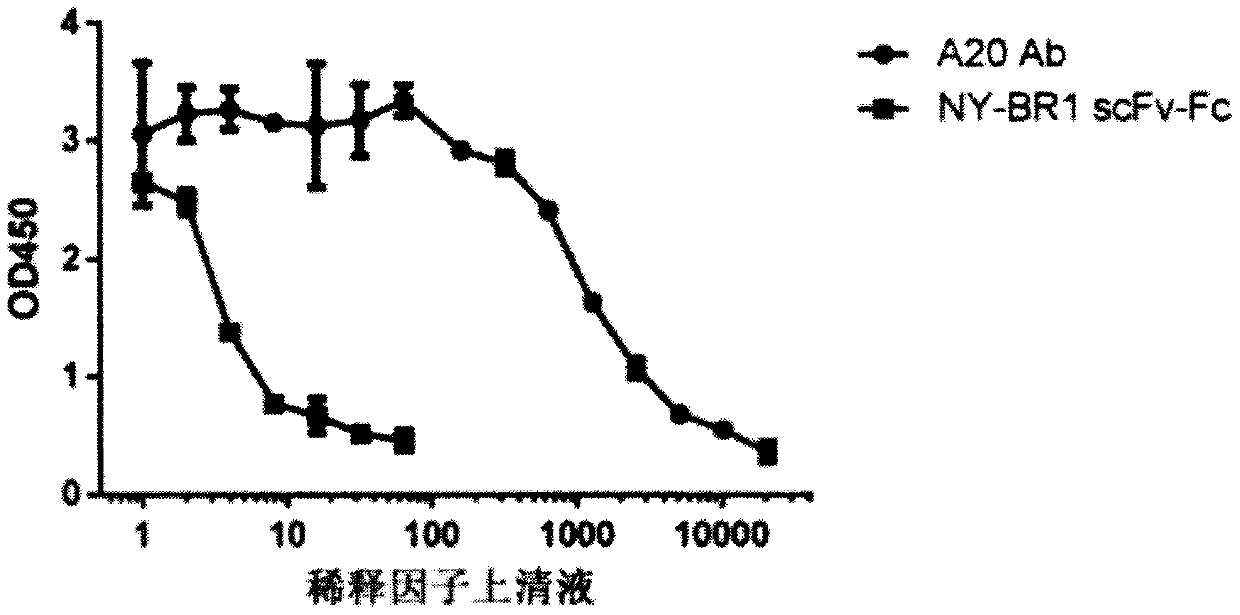
[0160] Example 2: Detection of NY-BR1-LCAR AAV by ELISA
[0161] Purified NY-BR1 LCAR AAV particles were coated onto well assay plate (half well). In 50 μl NaHCO at 4 °C 3 Coating was performed overnight in buffer / well. The starting concentration is 1x10 9Viral genome (VG) / ml, followed by serial 2-fold dilution of the pellet in 6 steps. After incubation, the plate was washed six times with PBS-T (1xPBS+0.05% Tween 20) at 150 μl / well. Blocking of the plate was performed by incubating for 1 hour at room temperature with 150 μl / well of blocking buffer (3% BSA, 5% sucrose in PBS-T) on a rocking device. Mouse primary antibody (NY-BR-1 No.2 - ThermoFisher order number: MA5-12645) or soluble LCAR (Morab2scFv - produced in-house) was added to each well at a final concentration of 1 μg / ml in 30 μl PBS-T, It was then incubated for 1 h at room temperature on a shaker. Thereafter, the cells were washed 3 times with PBS-T (150 µl / well). Add a secondary antibody relative to the pri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com