Lactobacillus paracasei CCFM1069 and application thereof
A Lactobacillus and paracheese technology, applied in the field of microorganisms, can solve the problems of non-long-term use, side effects, etc., and achieve the effects of strong adsorption capacity, alleviation of toxicity, and improvement of fecal water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
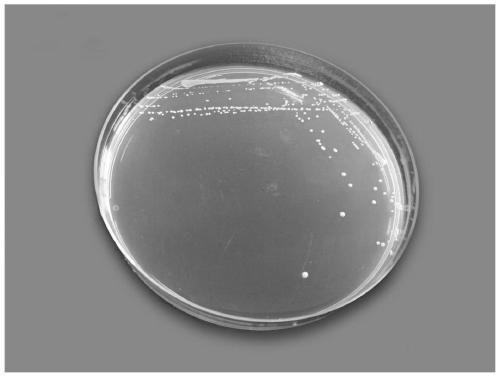
[0079] Embodiment 1: Lactobacillus paracasei CCFM1069 has good tolerance to simulated gastrointestinal fluid
[0080] The cryopreserved Lactobacillus paracasei CCFM1069 was inoculated in mMRS medium (MRS medium + 0.05% cysteine hydrochloride), cultured anaerobically at 37°C for 48 hours, and subcultured in mMRS medium for 2-3 hours. After the second time, take 1mL of the culture solution of Lactobacillus paracasei CCFM1069, mix it with 9.0mL of pH 2.5 artificial simulated gastric juice (containing 1% pepsin, mMRS medium at pH=2.5), and culture it anaerobically at 37°C. Samples were taken at 0h, 0.5h, 1h, and 2h, and the mMRS agar medium was poured to culture the plate colonies, and the number of viable bacteria was determined and the survival rate was calculated.
[0081] The survival rate is the ratio of the logarithmic value of the number of viable bacteria at the time of sampling to the logarithmic value of the number of viable bacteria at the 0th hour in the culture solu...
Embodiment 2
[0086] Embodiment 2: Lactobacillus paracasei CCFM1069 has no toxic and side effects to BALB / C mice
[0087] Suspend Lactobacillus paracasei CCFM1069 bacterium in 2% sucrose solution to make a concentration of 3.0 × 10 9 CFU / mL bacterial suspension. Eight healthy male BALB / C mice with a body weight of about 16-20 g were taken, and after one week of adaptation to the environment, they were given the bacterial suspension of this concentration by intragastric administration once a day, observed for one week, and the death and body weight were recorded.
[0088] The results of these tests are listed in Table 3. These results indicate that feeding concentrations of 3.0×10 9 CFU / mL of Lactobacillus paracasei CCFM1069 had no significant impact on mice, no significant change in body weight, and no death. The appearance of the mice had no obvious pathological symptoms.
[0089] Table 3 Changes in body weight and death of mice
[0090]
[0091] Note: -: no death of mice
Embodiment 3
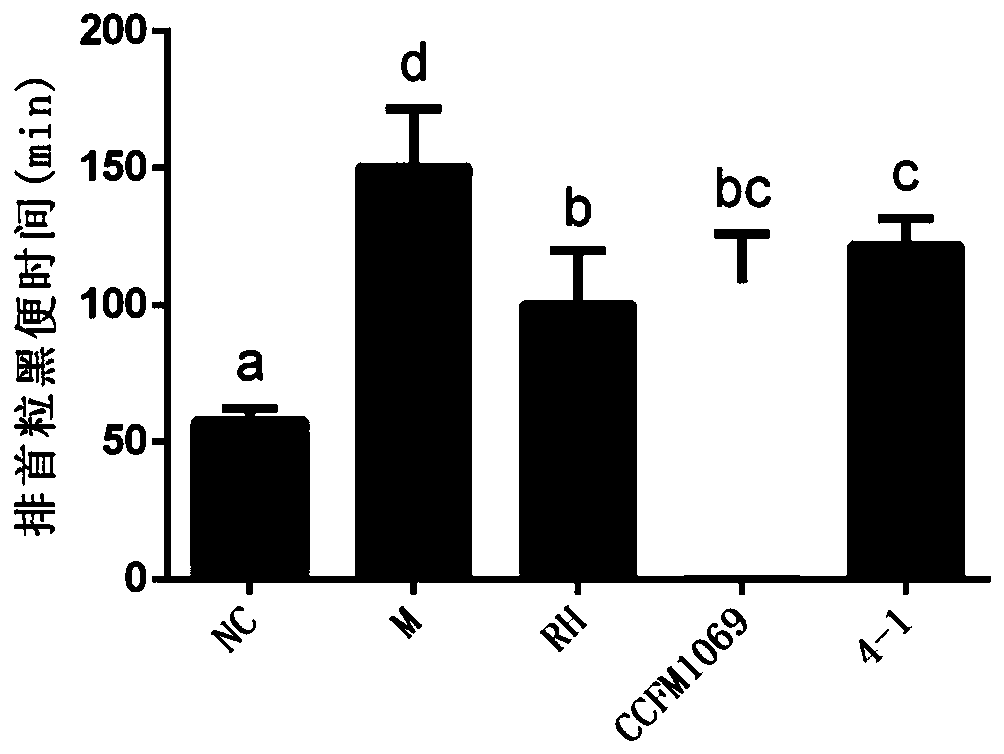
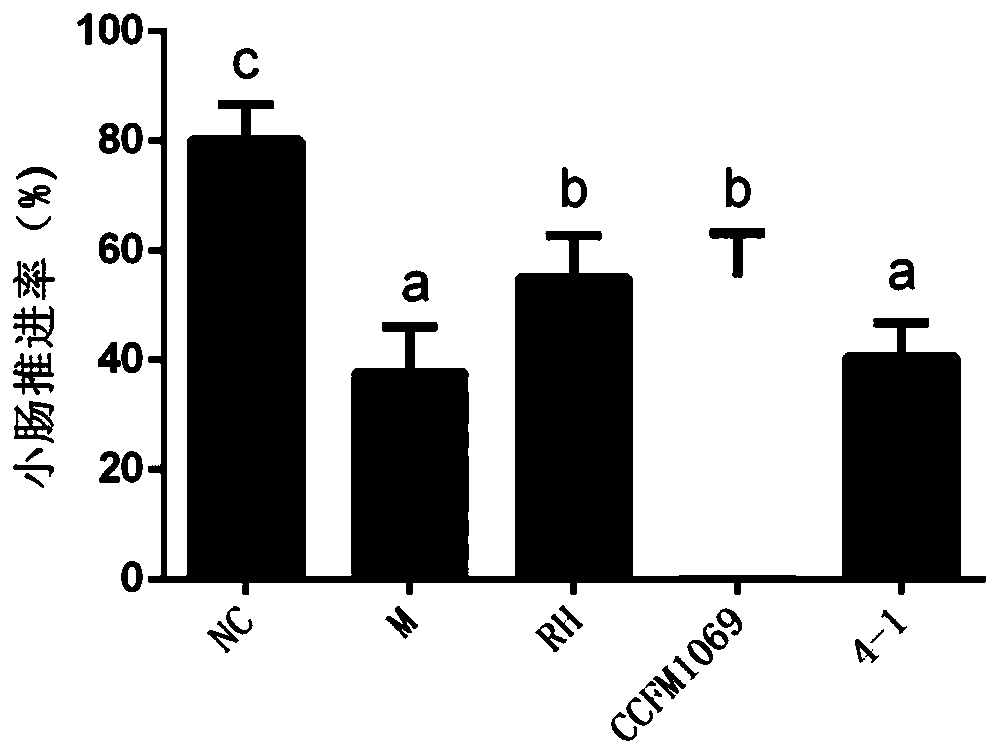
[0092] Embodiment 3: Effect of Lactobacillus paracasei CCFM1069 on the first black stool of constipation mice
[0093] Take Lactobacillus paracasei CCFM1069 out of the refrigerator at -80°C, streak on the MRS plate, culture at 37°C for 48 hours, pick a single colony in the MRS liquid tube, culture at 37°C for 18 hours, and inoculate at 2% volume In the new MRS liquid medium, culture at 37°C for 18h, culture for another generation in the same way, then centrifuge the lactic acid bacteria suspension at 8000r / min, 4°C for 8min, and then re-infect with 3% sucrose solution. Suspended to obtain a bacterial suspension, which was stored in a -80°C refrigerator.
[0094] Take 30 6-week-old healthy male Balb / C mice, acclimate to the environment for 1 week, and divide them into 5 groups randomly: blank control group (NC), model control group (M), phenolphthalein control group (RH), para-cheese milk Bacillus CCFM1069 intervention group (CCFM1060), Lactobacillus paracasei 4-1 (4-1), each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com